In the present manuscript, a brief overview on barium, its possible utilization, and the
aftermath of its behavior in organisms has been presented. A number of studies have exhibited
both the unwanted outcome barium displayed and the advantages of barium laden compounds,
tested in in vitro and in vivo settings. The plethora of prospective manipulations covered the area
of hydrogels and calcium phosphates, with an end goal of examining barium’s future in the tissue
engineering. Can barium be used as a substitute for other biologically relevant divalent cations? Will the incorporation of barium ions hamper the execution of the essential processes in the organism? Most importantly, can the benefits outweigh the harm?
- barium
- biomaterials
- hydrogels
- physiology
- bone tissue regeneration
- calcium phosphate
- Introduction
Scaffolds used in the bone tissue engineering have been under a continuous scrutiny. Establishing an ideal construct that corresponds to the set goals of biocompatibility, biodegradability, promotion of bone regeneration, while at the same time mimicking the distinctive properties of natural bone, has proven to be strenuous [1]. One of the constituents that have taken the spotlight of being the most promising ones are calcium phosphates (CaPs). The spectra of calcium phosphates encompasses twelve CaPs, with the Ca/P molar ratio ranging from 0.5 to 2.0 [2–4]. Particular relevance goes into the fact that they represent the inorganic part of bone and teeth[2], which is why they are closely mentioned with the process of biomineralization. “Biomineralization can be described as a phenomenon in which a mineral is integrated as a functional and often structural part of living organisms, often in direct and close contact to a matrix forming protein or carbohydrate structure” [5]. As a part of the bone, apatite is presumably formed from the non-stoichiometric and ion-doped CaPs, originating from amorphous form [2,6–8]. In a detailed in situ investigation, Habraken et al. [8] described the process starting from the generated pre-nucleation complexes, called Posner’s clusters, which essentially are calcium triphosphate ion-association complexes. Subsequent stage covered nucleation of amorphous calcium phosphate (ACP), with a following conversion to octacalcium phosphate (OCP), through a continuous binding of calcium ions (Ca2+). The postulated mechanism ends with the formation of apatite, embodying the calcium triphosphate complex as its fundamental structural unit. Well established crystal structure of CaPs, included within the ternary system Ca(OH)2-H3PO4-H2O, enables the transitions from one form to another (e.g. layer-by-Iayer growth mechanism of HAp through OCP), as well as numerous incorporations [9]. Functionalization of CaPs with miscellaneous ions has proven to be beneficial in diverse stages of bone regeneration processes (Figure 1). Up until now, multiple ions have been used to steer the pathways of complex mechanisms transpiring in the body. These ions range from vanadium (V5+), niobium (Nb5+), boron (B3+), gallium (Ga3+), iron (Fe3+), to calcium (Ca2+), cobalt (Co2+), copper(II) (Cu2+), magnesium (Mg2+), strontium (Sr2+), zinc (Zn2+), lithium (Li+), silver (Ag+), fluoride (F-), bromide (Br-), chloride (Cl-), hydroxyl (OH-), hydrogen phosphate (HPO42−), carbonate (CO32-), phosphate (PO43-) and silicate (Si4-)[10–17]. Cationic substitutions of CaP, e.g. HAp generalized through a formula M10(XO4)6Y2, where M is typically a bivalent cation, can occur with a complete or partial replacement of Ca2+[16]. Depending on an ionic radius and concentration, these substitutions can either stabilize the structure or destabilize the lattice [18,19].
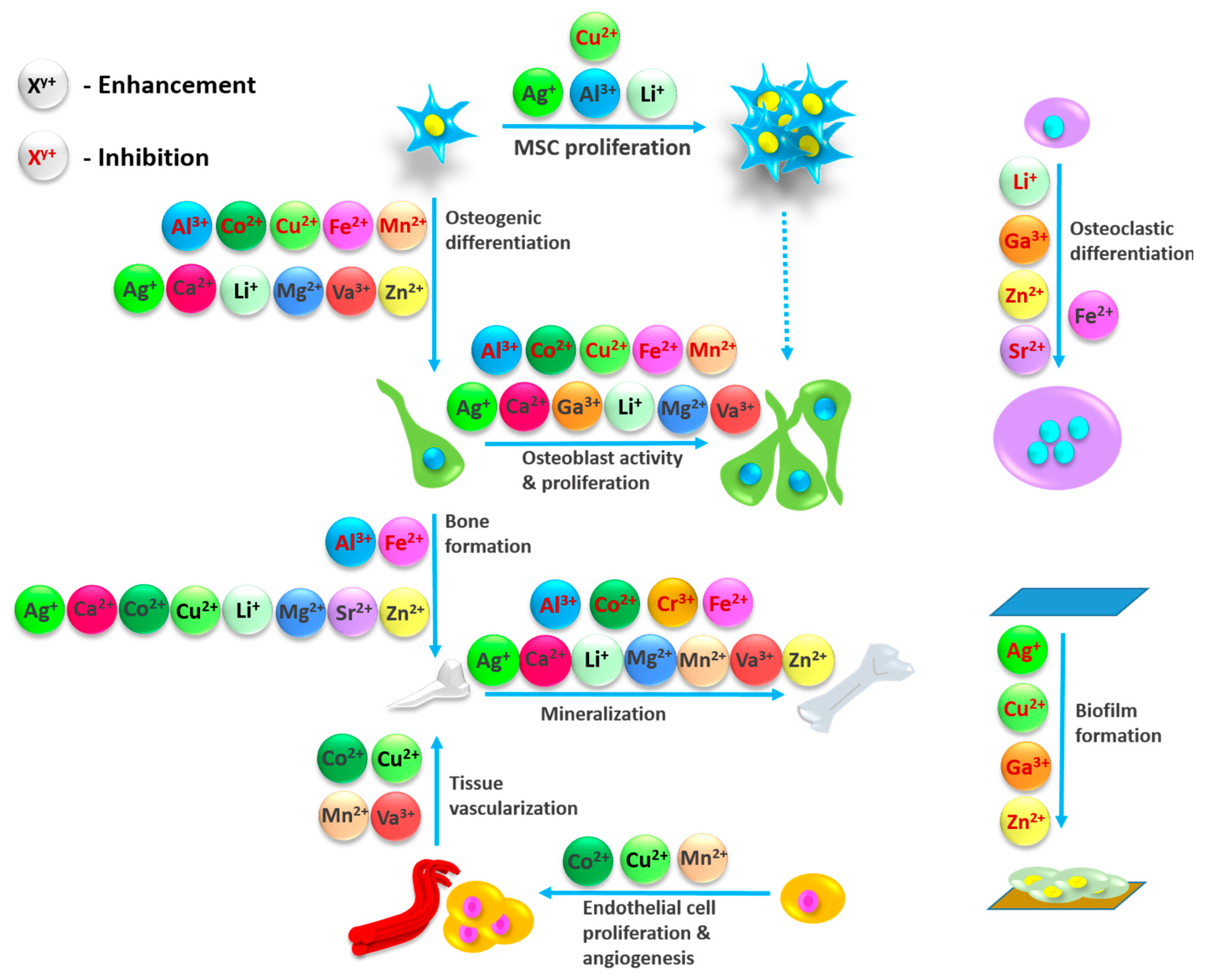
Figure 1. Effect different ions display in bone regeneration processes. Obtained from the reference [13]
Furthermore, ions have not only been used to ameliorate the structure, morphology and the effect CaPs have, but they have been used also as crosslinking agents for the hydrogels [20]. Hydrogels represent three dimensional hydrophilic polymer network with high affinity toward the water. Depending on their crosslinking approach (chemical or physical), properties like reduced dissolution, or distinctive mechanical and biochemical properties, with various functions (e.g. incorporation of 0.61 wt% of Zn2+ in HAp resulted in the extension of their lag time increasing its antibacterial potential) can materialize [24]. One of the physical approaches of hydrogel crosslinking is with ionic/electrostatic interactions (more information in section 3.1) [21]. Even though large spectra of studies have examined the influence of bivalent cations such as Mg2+[22], Sr2+ [23]and Zn2+[24], there is a scarcity of information regarding the effect of one more alkaline earth metal – barium (Ba2+). Barium’s participation in bone repair and regeneration has placed it in the forefront of recent interest. In nature, barium does not occur in its free ionic form, but as a number of natural salt compounds e.g., barite (BaSO4) or barium chloride (BaCl2) [25]. Barium compounds that are relatively soluble in water are acetate, nitrate, and halides (except fluoride), while compounds with carbonate, chromate, fluoride, oxalate, phosphate, and sulphate are fairly insoluble in water (Table 1) [26]. Bearing the benefits of the ion incorporation in mind, a question arises – why has not the influence of barium on CaPs and their composites been examined more in depth?
- Barium comprising biomaterials and their biological performance
Despite the fact that barium, as a divalent cation, has a vast potential to be utilized in combination with biologically relevant biomaterials, the mechanism of apposition or the outcome of possible effects is insufficiently researched. Detailed review of literature has pointed out that when Ba2+ was combined with pertinent polymers (e.g. alginate or hyaluronic acid), it elucidated promising results. Considerably smaller number of studies has underlined the ramifications of the Ba2+ – CaP fusion. Nevertheless, positive data regarding mechanical properties and biocompatibility has been presented.
3.1. Barium loaded hydrogels
Hydrogels are hydrophilic, polymer-based systems that absorb and preserve large amounts of water [54,55]. When making hydrogels, certain sort of a crosslink is formed, whether through chemical crosslinking (covalent or ionic bonds) or physical crosslinking (ionic forces or electrostatic forces). In addition, van der Waals forces and hydrogen bonds can also operate as crosslinks [56].
One of the ways physically crosslinked hydrogels can be synthesized is through the interplay of various ions at mild conditions (room temperature and physiological pH). A hydrogel with stronger properties will be achieved by using metallic ions due to the coordination stemming from Lewis acid–base interactions [54,55]. Commonly, the most explored hydrogels, crosslinked with metal ions, are the ones with coordination tethered by metal cations [57]. For that purpose, such cations as Fe3+, Ca2+, Sr2+ and Zn2+ are widely used. Barium ions have the ability to form salts with particularly low solubility in aqueous media. On this accord, several studies [58–63] have examined the effect of barium crosslinking on the overall properties of different polymers. As a divalent cation, Ba2+ usually forms ionic crosslinks, which transpire as a prerequisite of achieving electrical neutrality in the material [58]. Barium has the capability to establish two crosslinking mechanisms within the materials, already mentioned ionic crosslinks, and physical crosslinks[58]. Ionic are independent of temperature, while physically crosslinked materials are supposed to be temperature – dependent. Further distinction between these two mechanisms is that physically crosslinked materials are formed owing to ion–dipole associations of the BaSO4 groups, producing ionic aggregation i.e., ion-clusters. Ion-clusters secure versatile crosslinks constructed by nano-phase separation of ion-rich domains (1–5nm). In order to prove which crosslinking transpired, structural analysis is required.
In the study conducted by Gasa et al., barium was used on acidic polymer electrolyte membranes (PEM), based on sulfonated poly(ether ketone ketone) (SPEKK), so as to reduce the sorption of aqueous media and consequentially improve their mechanical properties and stability [58]. The crosslinking between sulphonate groups occurred by the exchange of barium ions with the protons in SPEKK membranes. Increase of the exchanged barium resulted in the decrease of equilibrium water sorption (17wt%). However, when the Ba2+ exchange was above 64%, the fluid uptake was practically independent of temperature and methanol activity in water-ethanol solutions. Nonetheless, if the percentage of exchanged cation was lower, the temperature dependence was visible (<45 ̊C weak dependence, >45 ̊C sharp upturn in the water sorption). The reason for this behavior is most likely the glass-to-rubbery state transition of the water-swollen SPEKK. Moreover, thermal stability was considerably improved in dry conditions. As it was mentioned before, size of the barium ions is substantial in comparison to others, hence they exhibit less mobility than the mobile protons that were interchanged. When combined with the partially deprotonated hyaluronic acid, barium (similar to other bivalent cations) results in the formation of chelate-like complexes, followed by an increasing degree of cross-linking within or between polymer chains [59]. The viscosity of the hyaluronate solution was substantially lowered with an increase of cation concentration, while the conformation was radically changed.
Conversely, the highest number of papers was associated with crosslinking of barium and alginate [60,62,64–68].
The “egg box model” is commonly used to describe the formation of alginate gels. The divalent ions interacted jointly with G blocks to form ionic bridges between adjoining chains [62,71].
Due to this specific binding and the size of the ion itself, barium crosslinked gel manifested lower swelling degree, thus, it was more stable in aqueous media [62]. In a study by Bajpai et al.[66,72], alginate formed beads were placed in a buffer medium with pH 7.4. Barium ions bounded to the carboxylic (COO-) groups, starting the process of exchange with sodium ions situated in the swelling medium. After the maximum swelling of the beads was achieved, barium ions in the egg-box junctions started to diffuse out and the beads began slowly disintegrating in a longer period of time owing to the ion size [66].
The paucity of information regarding the association of barium and different types of polymers, and their effect on biological performance can be credited to only several papers, dating back to 1990s [74–76]. However, even with promising results several of these groups obtained, no detailed work on further barium use was performed.
3.2. Synthesis of calcium phosphates containing barium
As a divalent cation, barium extends the possibility of being incorporated within different calcium phosphates. There have been few studies concerning the preparation of barium–calcium apatites [77–81]. Bigi et al., have tried to form barium–calcium hydroxyapatite (BaCaHAp) by a solid state reaction at 1200°C and by a precipitation method at 100°C [82]. The products obtained by the solid state reaction, at high temperatures, covered the array of barium concentrations from 0 to 100 atom%. By using that method, lattice dimensions and the FT-IR absorption frequencies displayed linear increase, following the increase of atom% of Ba2+. Only small quantities of Ba2+ were incorporated in HAp by precipitation from the aqueous system. Liu et al., synthesized calcium phosphate cement (CPC) powder with a mixture of α-tricalcium phosphate (α-TCP) and dicalcium phosphate dihydrate (DCPD) at the mass ratio of 9:1, with the addition of 20wt% starch and 20wt% BaSO4 [80]. Their aim was to look into the effects of BaSO4 on injectability and radiopacity, as well as the mechanical and biocompatibility properties of the CPC system. The compressive strength of the construct increased to over 50 MPa, with the injectability index higher than 90% (50 N at a constant injection speed of approximately 10 mm/min). In addition, the recorded radiopacity was high, while the setting times and biodegradation behaviour was satisfying. Moreover, in vitro tests on hemolysis, endotoxins and apoptosis, as well as subcutaneous implantation in vivo, demonstrated that the barium laden cement was nontoxic and biocompatible. In an another example of doping α-TCP with Ba2+ [83], stoichiometric amounts of ammonium dihydrogen phosphate (NH4H2PO4) and barium carbonate (BaCO3) were used with an end product of Ba-substituted α-TCP, (Ca1-xBax)3(PO4)2 (x = 0.05, 0.10, and 0.15). The results showed that the unit-cell volumes of the product were larger than that of undoped product (undoped a=12.87271 Å, b=27.28034 Å, c=15.21275 Å; doped a=13.0965 Å, b=27.9046 Å, c=15.4021 Å), which would suggest that the reactivity of barium doped α-TCP is higher. Yasukawa and his team synthesized carbonated BaCaHAp solid solution, with different Ba/(Ba + Ca) (XBa) atomic ratios (0-1), using the wet precipitation method, at 100oC [79]. Their results showed that no pure BaCaHAp was able to form, due to the irreversible adsorption amount of carbon dioxide (CO2). However, it should be noted that the information on substitution efficiency of barium was not mentioned in the study. Yoder et al., synthesized carbonated barium hydroxylapatite (CBaApOH) and carbonated barium chlorapatite (CBaApCl) by aqueous synthesis. The end goal was to define the mechanism of carbonate substitution at 60 or 90 °C, as before it was only preformed at solid state, high-temperature synthesis [78]. Their main conclusions were that the synthesis parameters had to be closely monitored to avoid the precipitation of simple salts (BaCO3, Ba3(PO4)2 and BaApCl), mainly because of their close molar solubilities. CBaApCl and CBaApOH demonstrated solubilities that are marginally higher than the solubilities of their noncarbonated analogs, at low carbonate concentrations.
- Biological influence of barium
Several studies have reported that barium laden materials provide a favourable environment for the cells and array of divergent functions [68]. The actual data collected on the overall biological influence of barium, incorporated in various calcium phosphates and hydrogels, is still scarce.
Three independent studies have also underlined the role of barium incorporation as an ameliorating component for drug delivery systems [61,72,87].
- Barium toxicity
Even with a high potential of being a good substitute for commonly used metallic ions, barium has certain downsides. Knowing the current data available, and the fact that barium is known to be toxic, approach to using barium as a crosslinking agent is still used with precaution [26,44,50]. Causes for individuals sensitivity to barium toxicity, as well as its role on epigenetic factors, are correlated with specific geographic/geological areas, and the distributed information are quite limited [25]. In order to avoid these ramifications, extensive studies with different approaches are needed. For example, studies on leakage of alginate gels, crosslinked with Ba2+ ions, have shown that when using low concentrations and vigorous rinsing of barium beads, there is no leakage of the ion, hence no repercussions [64,88].
- Future directions and conclusions
Albeit being one of the metallic ions, with all the characteristics ascribed to them (ionic radii, solubility, oxidation number etc.), barium has not gone the comprehensive and diverse research. Most of the findings and knowhow on barium have their origin in the second half of the 20th century, with scarce follow up in the recent years.
In the previous sections, we presented a brief outlook on barium itself, its integration within hydrogels and its potential to be merged with biologically relevant calcium phosphates (Figure 6). Biological influence and toxicity assessment has put barium in the shadow of the other important ions such as calcium, strontium and zinc. Several sources have underlined the negative side of barium in the organism – potential toxicity, blocking of potassium channels, lowering of cell viability etc. However, the positive results should not be merely side-lined. Crosslinking of barium and polymers resulted in stronger matrix, lower swelling degree, tighter formation and higher water resistance. As for the biological ramifications, islets embedded in microcapsules containing barium were able to reverse diabetes for almost a year. Furthermore, the cell lines CCL-13 and L929 grew rapidly and reached confluence after three days on barium crosslinked matrix. Once barium was combined with calcium phosphates, such as HAp and α-TCP, obtained cements exhibited nontoxicity and biocompatibility, with faster setting time. Moreover, in a separate study using akermanite as a starting point, the increase of Ba2+ ratio increased the ability to form apatite.
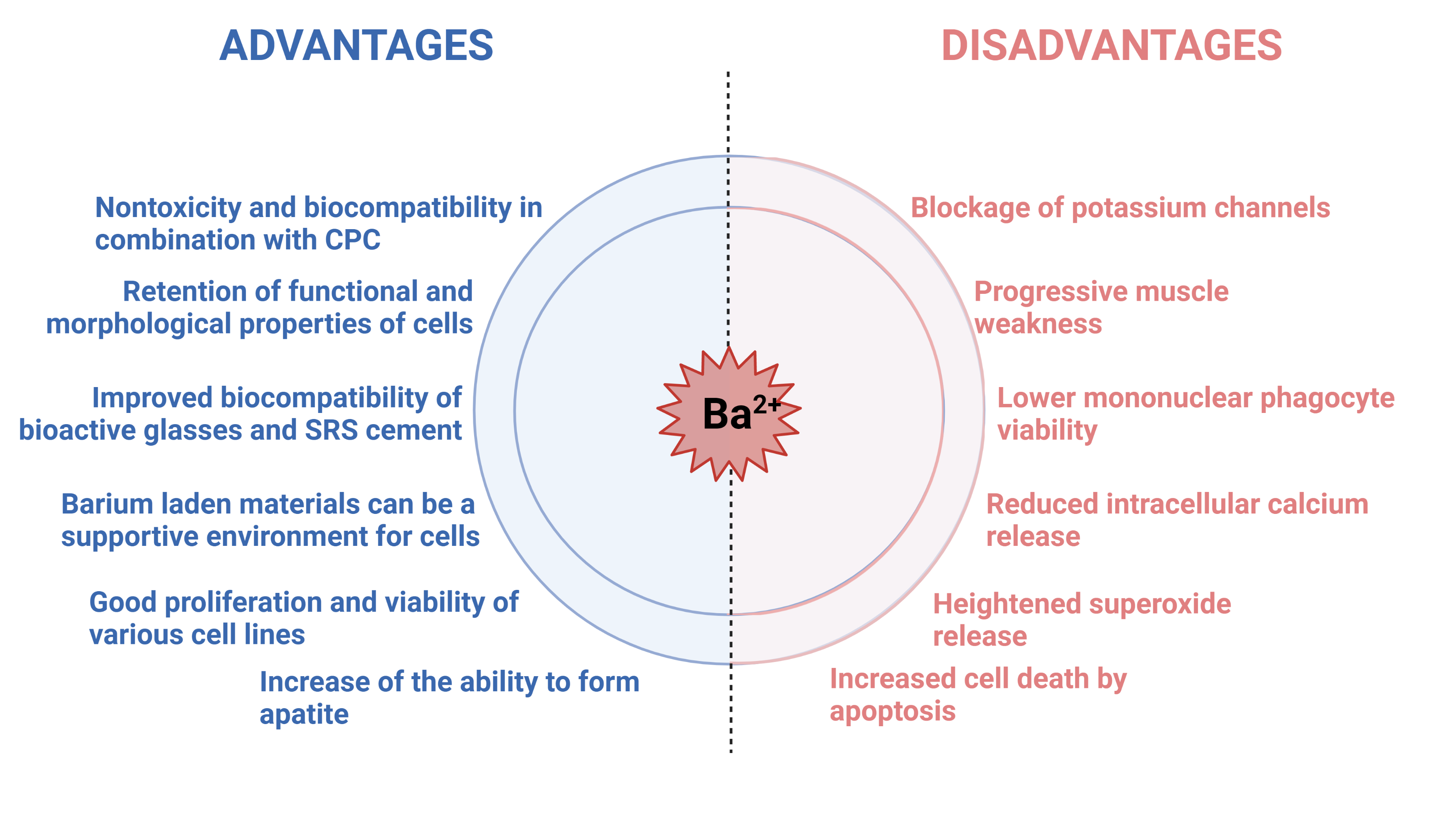
Figure 6. Brief overview of effects recorded in presence of barium ions in in vitro and in vivo setting. Figure created in BioRender.com
Bearing all the information in mind, it must be emphasized that the toxic effects were for oral or intravenous administration of barium containing matrices. Consequently, the limited research on local barium influence on cells or antimicrobial properties should be further explored, as the shown potential and possibly much lower administrated dose rate are one of the important factors. Added deduction that stemmed from the thorough literature search is that the studies performed on barium incorporation, effects and influence, are outdated. A fresh outlook on the overall behaviour of barium and barium loaded compounds is of vital importance. Use of the state-of-the-art equipment and newly established methodologies will yield new discoveries and help to clarify the potential benefits that barium has to offer in the field of bone tissue regeneration and possibly propel barium in the forefront of science.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91]
This entry is adapted from the peer-reviewed paper 10.3390/ma14195772
References
- [1] S.T. Bendtsen, M. Wei, Synthesis and characterization of a novel injectable alginate–collagen–hydroxyapatite hydrogel for bone tissue regeneration, J. Mater. Chem. B. (2015). https://doi.org/10.1039/C5TB00072F.
- [2] S. V. Dorozhkin, Calcium orthophosphates (CaPO4): occurrence and properties, 2016. https://doi.org/10.1007/s40204-015-0045-z.
- [3] S. V Dorozhkin, M. Epple, Biological and Medical Significance of Calcium Phosphates, Angew. Chem. Int. Ed. 41 (2002) 3130–3146.
- [4] S. V. Dorozhkin, A review on the dissolution models of calcium apatites, Prog. Cryst. Growth Charact. Mater. 44 (2002) 45–61. https://doi.org/10.1016/S0960-8974(02)00004-9.
- [5] W. Habraken, P. Habibovic, M. Epple, M. Bohner, Calcium phosphates in biomedical applications: Materials for the future?, Mater. Today. 19 (2016) 69–87. https://doi.org/10.1016/j.mattod.2015.10.008.
- [6] S. V. Dorozhkin, Calcium orthophosphates and human beings: a historical perspective from the 1770s until 1940., Biomatter. 2 (2012) 53–70. https://doi.org/10.4161/biom.21340.
- [7] S. V. Dorozhkin, A hierarchical structure for apatite crystals, J. Mater. Sci. Mater. Med. 18 (2007) 363–366. https://doi.org/10.1007/s10856-006-0701-x.
- [8] W.J.E.M. Habraken, J. Tao, L.J. Brylka, H. Friedrich, L. Bertinetti, A.S. Schenk, A. Verch, V. Dmitrovic, P.H.H. Bomans, P.M. Frederik, J. Laven, P. Van Der Schoot, B. Aichmayer, G. De With, J.J. DeYoreo, N.A.J.M. Sommerdijk, Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate, Nat. Commun. 4 (2013) 1507–1512. https://doi.org/10.1038/ncomms2490.
- [9] M.S. Tung, Calcium Phosphates: Structure, Composition, Solubility, and Stability, Calcium Phosphates Biol. Ind. Syst. (1998) 1–19. https://doi.org/10.1007/978-1-4615-5517-9_1.
- [10] C. Garbo, J. Locs, M. D’este, G. Demazeau, A. Mocanu, C. Roman, O. Horovitz, M. Tomoaia-Cotisel, Advanced Mg, Zn, Sr, Si multi-substituted hydroxyapatites for bone regeneration, Int. J. Nanomedicine. 15 (2020) 1037–1058. https://doi.org/10.2147/IJN.S226630.
- [11] V. Mouriño, J.P. Cattalini, A.R. Boccaccini, Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments, J. R. Soc. Interface. 9 (2012) 401–419. https://doi.org/10.1098/rsif.2011.0611.
- [12] A. Dubnika, D. Loca, V. Rudovica, M.B. Parekh, L. Berzina-Cimdina, Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery, Ceram. Int. 43 (2017) 3698–3705. https://doi.org/10.1016/j.ceramint.2016.11.214.
- [13] K. Glenske, P. Donkiewicz, A. Köwitsch, N. Milosevic-Oljaca, P. Rider, S. Rofall, J. Franke, O. Jung, R. Smeets, R. Schnettler, S. Wenisch, M. Barbeck, Applications of metals for bone regeneration, 2018. https://doi.org/10.3390/ijms19030826.
- [14] S.H. Lin, W.J. Zhang, X.Q. Jiang, Applications of Bioactive Ions in Bone Regeneration, Chin. J. Dent. Res. 22 (2019) 93–104. https://doi.org/10.3290/j.cjdr.a42513.
- [15] A. Laskus, J. Kolmas, Ionic substitutions in non-apatitic calcium phosphates, Int. J. Mol. Sci. 18 (2017) 1–22. https://doi.org/10.3390/ijms18122542.
- [16] E. Boanini, M. Gazzano, A. Bigi, Ionic substitutions in calcium phosphates synthesized at low temperature, Acta Biomater. 6 (2010) 1882–1894. https://doi.org/10.1016/j.actbio.2009.12.041.
- [17] E. O’Neill, G. Awale, L. Daneshmandi, O. Umerah, K.W.H. Lo, The roles of ions on bone regeneration, Drug Discov. Today. 23 (2018) 879–890. https://doi.org/10.1016/j.drudis.2018.01.049.
- [18] H. Shi, X. Ye, T. Wu, J. Zhang, J. Ye, Regulating the physicochemical and biological properties in vitro of octacalcium phosphate by substitution with strontium in a large doping range, Mater. Today Chem. 5 (2017) 81–91. https://doi.org/10.1016/j.mtchem.2017.07.003.
- [19] H. Shi, F. He, J. Ye, Synthesis and structure of iron- and strontium-substituted octacalcium phosphate: Effects of ionic charge and radius, J. Mater. Chem. B. 4 (2016) 1712–1719. https://doi.org/10.1039/c5tb02247a.
- [20] A.I. Sarker, M. Izadifar, D. Schreyer, Influence of ionic cross linkers ( Ca / Ba / Zn ) on the Mechanical and Biological Properties of 3D Bioplotted Hydrogel Scaffolds, J. Biomater. Sci. Polym. Ed. 5063 (2018) 0–1. https://doi.org/10.1080/09205063.2018.1433420.
- [21] W. Hu, Z. Wang, Y. Xiao, S. Zhang, J. Wang, Advances in crosslinking strategies of biomedical hydrogels, Biomater. Sci. (2019). https://doi.org/10.1039/c8bm01246f.
- [22] I. Akbar, S. Kim, Characteristic of magnesium substituted octacalcium phosphate prepared by precipitation method, AIP Conf. Proc. 2092 (2019). https://doi.org/10.1063/1.5096677.
- [23] L. Stipniece, K. Salma-Ancane, D. Loca, S. Pastare, Synthesis of strontium substituted hydroxyapatite through different precipitation routes, Key Eng. Mater. 674 (2016) 3–8. https://doi.org/10.4028/www.scientific.net/KEM.674.3.
- [24] N. Strutynska, O. Livitska, S. Prylutska, Y. Yumyna, P. Zelena, L. Skivka, A. Malyshenko, L. Vovchenko, V. Strelchuk, Y. Prylutskyy, N. Slobodyanik, U. Ritter, New nanostructured apatite-type (Na+,Zn2+,CO32−)-doped calcium phosphates: Preparation, mechanical properties and antibacterial activity, J. Mol. Struct. 1222 (2020) 128932. https://doi.org/10.1016/j.molstruc.2020.128932.
- [25] J. Kravchenko, T.H. Darrah, R.K. Miller, H.K. Lyerly, A. Vengosh, A review of the health impacts of barium from natural and anthropogenic exposure, Environ. Geochem. Health. 36 (2014) 797–814. https://doi.org/10.1007/s10653-014-9622-7.
- [26] J. Colman, L. Ingerman, P. Robbins, TOXICOLOGICAL REVIEW OF BARIUM AND COMPOUNDS, Support Summ. Inf. Integr. Risk Inf. Syst. (2005).
- [27] T.R. Harring, N.S. Deal, D.C. Kuo, Disorders of sodium and water balance, Emerg. Med. Clin. North Am. 32 (2014) 379–401. https://doi.org/10.1016/j.emc.2014.01.001.
- [28] G. Kaur, N. Kaur, Estimation of sodium ions using easily engineered organic nanoparticles-based turn-on fluorescent sensor: Application in biological and environmental samples, Sensors Actuators, B Chem. 265 (2018) 134–141. https://doi.org/10.1016/j.snb.2018.02.063.
- [29] M. Vašák, J. Schnabl, Sodium and Potassium Ions in Proteins and Enzyme Catalysis, in: Alkali Met. Ions Their Role Life, 2016: pp. 485–556. https://doi.org/10.1007/978-3-319-21756-7.
- [30] N.T. Hideki Sakai , Takuto Fujii, Proton-Potassium (H + /K + ) ATPases: Properties and Roles in Health and Diseases, in: Alkali Met. Ions Their Role Life, 2016: pp. 485–556. https://doi.org/10.1007/978-3-319-21756-7.
- [31] X. Pang, L. Lin, B. Tang, Unraveling the role of Calcium ions in the mechanical properties of individual collagen fibrils, Sci. Rep. 7 (2017) 1–8. https://doi.org/10.1038/srep46042.
- [32] J.A. Beto, The Role of Calcium in Human Aging, Clin. Nutr. Res. 4 (2015) 1–8. https://doi.org/10.7762/cnr.2015.4.1.1.
- [33] S. Choi, K.J. Kim, S. Cheon, E.M. Kim, Y.A. Kim, C. Park, K.K. Kim, Biochemical activity of magnesium ions on human osteoblast migration, Biochem. Biophys. Res. Commun. 531 (2020) 588–594. https://doi.org/10.1016/j.bbrc.2020.07.057.
- [34] T. Qi, J. Weng, F. Yu, W. Zhang, G. Li, H. Qin, Z. Tan, H. Zeng, Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells, Biol. Trace Elem. Res. 199 (2020) 559–567. https://doi.org/10.1007/s12011-020-02183-y.
- [35] D. Loca, A. Smirnova, J. Locs, A. Dubnika, J. Vecstaudza, L. Stipniece, E. Makarova, M. Dambrova, Development of local strontium ranelate delivery systems and long term in vitro drug release studies in osteogenic medium, Sci. Rep. 8 (2018) 1–10. https://doi.org/10.1038/s41598-018-35197-7.
- [36] M. Pilmane, K. Salma-Ancane, D. Loca, J. Locs, L. Berzina-Cimdina, Strontium and strontium ranelate: Historical review of some of their functions, Mater. Sci. Eng. C. 78 (2017) 1222–1230. https://doi.org/10.1016/j.msec.2017.05.042.
- [37] W.Y. Chan, O.M. Rennert, The role of copper in iron metabolism, Ann. Clin. Lab. Sci. 10 (1980) 338–344.
- [38] J. Osredkar, N. Sustar, Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance, J Clin. Toxicol. 3 (2011). https://doi.org/http://dx.doi.org/10.4172/2161-0494.S3-001.
- [39] J.E. Cummings, J.P. Kovacic, The ubiquitous role of zinc in health and disease, J. Vet. Emerg. Crit. Care. 19 (2009) 215–240. https://doi.org/10.1111/j.1476-4431.2009.00418.x.
- [40] R.P.L. van Swelm, J.F.M. Wetzels, D.W. Swinkels, The multifaceted role of iron in renal health and disease, Nat. Rev. Nephrol. 16 (2020) 77–98. https://doi.org/10.1038/s41581-019-0197-5.
- [41] D.C.P. Gupta, Role of Iron (Fe) in Body, IOSR J. Appl. Chem. 7 (2014) 38–46. https://doi.org/10.9790/5736-071123846.
- [42] T. Theophanides, Metal Ions in Biological Systems, Int. J. Quantum Chem. 26 (1984) 933–941. https://doi.org/10.1111/j.1469-185X.1953.tb01384.x.
- [43] A. Sigel, H. Sigel, R.K.O. Sigel, The Alkali Metal Ions: Their Role for Life, 2016. https://doi.org/10.1007/978-3-319-21756-7_12.
- [44] Henry A. Schroeder, I.H. Tipton, A.P. Nason, TRACE METALS IN MAN : STRONTIUM AND BARIUM *, J Chron Dis. 25 (1972) 491–517.
- [45] A. Fischer, P. Malara, D. Wiechuła, The Study of Barium Concentration in Deciduous Teeth, Impacted Teeth, and Facial Bones of Polish Residents, Biol. Trace Elem. Res. 161 (2014) 32–37. https://doi.org/10.1007/s12011-014-0061-1.
- [46] C. Austin, T.M. Smith, A. Bradman, K. Hinde, R. Joannes-Boyau, D. Bishop, D.J. Hare, P. Doble, B. Eskenazi, M. Arora, Barium distributions in teeth reveal early-life dietary transitions in primates, Nature. 498 (2013) 216–219. https://doi.org/10.1038/nature12169.
- [47] E.M. SOWDEN, S.R. STITCH, Trace elements in human tissue. 2. Estimation of the concentrations of stable strontium and barium in human bone., Biochem. J. 67 (1957) 104–109. https://doi.org/10.1042/bj0670104.
- [48] A. Panahifar, L.D. Chapman, L. Weber, N. Samadi, D.M.L. Cooper, Biodistribution of strontium and barium in the developing and mature skeleton of rats, J. Bone Miner. Metab. 37 (2019) 385–398. https://doi.org/10.1007/s00774-018-0936-x.
- [49] P.H. Bligh, D.M. Taylor, Comparative Studies of the Metabolism of Strontium and Barium in the Rat, Biochem. J. 87 (1963) 612–617.
- [50] D. Moffet, C. Smith, Y. Stevens, L. Ingerman, S. Swarts, L. Chappell, Toxicological profile for barium and barium compounds, Agency Toxic Subst. Dis. Regist. (2007) 1–231. https://www.atsdr.cdc.gov/toxprofiles/tp24.pdf%0Ahttp://stacks.cdc.gov/view/cdc/6955/.
- [51] M. Peana, S. Medici, M. Dadar, M.A. Zoroddu, A. Pelucelli, C.T. Chasapis, G. Bjørklund, Environmental barium: potential exposure and health-hazards, Arch. Toxicol. (2021). https://doi.org/10.1007/s00204-021-03049-5.
- [52] A. Panahifar, T.M. Swanston, M. Jake Pushie, G. Belev, D. Chapman, L. Weber, D.M.L. Cooper, Three-dimensional labeling of newly formed bone using synchrotron radiation barium K-edge subtraction imaging, Phys. Med. Biol. 61 (2016) 5077–5088. https://doi.org/10.1088/0031-9155/61/13/5077.
- [53] A.D. Foster, The impact of bipedal mechanical loading history on longitudinal long bone growth, PLoS One. 14 (2019) 1–20. https://doi.org/10.1371/journal.pone.0211692.
- [54] W.E. Hennink, C.F. van Nostrum, Novel crosslinking methods to design hydrogels, Adv. Drug Deliv. Rev. 64 (2012) 223–236. https://doi.org/10.1016/j.addr.2012.09.009.
- [55] J. Maitra, V.K. Shukla, Cross-linking in Hydrogels - A Review, Am. J. Polym. Sci. 4 (2014) 25–31. https://doi.org/10.5923/j.ajps.20140402.01.
- [56] N.A. Peppas, Hydrogels in medicine and pharmacy, 2019. https://doi.org/10.1016/0168-3659(89)90068-0.
- [57] H. Li, P. Yang, P. Pageni, C. Tang, Recent Advances in Metal-Containing Polymer Hydrogels, Macromol. Rapid Commun. 38 (2017) 1–9. https://doi.org/10.1002/marc.201700109.
- [58] J. V Gasa, R.A. Weiss, M.T. Shaw, Ionic crosslinking of ionomer polymer electrolyte membranes using barium cations, J. Memb. Sci. 304 (2007) 173–180. https://doi.org/10.1016/j.memsci.2007.07.031.
- [59] A. Zellermann, D. Bergmann, C. Mayer, Cation induced conformation changes in hyaluronate solution, Eur. Polym. J. 49 (2013) 70–79. https://doi.org/10.1016/j.eurpolymj.2012.09.025.
- [60] A. Dodero, L. Pianella, S. Vicini, M. Alloisio, M. Ottonelli, M. Castellano, Alginate-based hydrogels prepared via ionic gelation : An experimental design approach to predict the crosslinking degree, Eur. Polym. J. 118 (2019) 586–594. https://doi.org/10.1016/j.eurpolymj.2019.06.028.
- [61] R.T. Thimma, S. Tammishetti, Barium Chloride Crosslinked Carboxymethyl Guar Gum Beads for Gastrointestinal Drug Delivery, J. Appl. Polym. Sci. 82 (2001) 3084–3090. https://doi.org/10.1002/app.2164.
- [62] A.C.K. Bierhalz, M.A. da Silva, M.E.M. Braga, H.J.C. Sousa, T.G. Kieckbusch, Effect of calcium and/or barium crosslinking on the physical and antimicrobial properties of natamycin-loaded alginate films, LWT - Food Sci. Technol. 57 (2014) 494–501. https://doi.org/10.1016/j.lwt.2014.02.021.
- [63] G. Li, G. Zhang, R. Sun, C. Wong, Mechanical strengthened alginate / polyacrylamide hydrogel crosslinked by barium and ferric dual ions, J. Mater. Sci. (2017). https://doi.org/10.1007/s10853-017-1066-x.
- [64] P. De Vos, M.M. Faas, B. Strand, R. Calafiore, Alginate-based microcapsules for immunoisolation of pancreatic islets, Biomaterials. 27 (2006) 5603–5617. https://doi.org/10.1016/j.biomaterials.2006.07.010.
- [65] G. Luca, M. Calvitti, D. Ph, C. Nastruzzi, D. Ph, L. Bilancetti, D. Ph, E. Becchetti, D. Ph, G. Angeletti, F. Mancuso, D. Ph, R. Calafiore, L.E.T. Al, Encapsulation, In Vitro Characterization, and In Vivo Biocompatibility of Sertoli Cells in Alginate-Based Microcapsules, Tissue Eng. 13 (2007). https://doi.org/10.1089/ten.2006.0137.
- [66] S.K. Bajpai, S. Sharma, Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions, React. Funct. Polym. 59 (2004) 129–140. https://doi.org/10.1016/j.reactfunctpolym.2004.01.002.
- [67] H. Alizadeh Sardroud, S. Nemati, A. Baradar Khoshfetrat, M. Nabavinia, Y. Beygi Khosrowshahi, Barium-cross-linked alginate-gelatine microcapsule as a potential platform for stem cell production and modular tissue formation, J. Microencapsul. 34 (2017) 488–497. https://doi.org/10.1080/02652048.2017.1354940.
- [68] I. Machida-sano, M. Hirakawa, H. Namiki, Cell Compatibility of Three-Dimensional Porous Barium-Cross-Linked Alginate Hydrogels, JSRR. 3 (2014) 2611–2621.
- [69] O. Smidsrød, Molecular basis for some physical properties of alginates in the gel state, Faraday Discuss. Chem. Soc. 57 (1974) 263.
- [70] Ý.A. Mørch, I. Donati, B.L. Strand, G. Skjåk-Bræk, Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads, Biomacromolecules. 7 (2006) 1471–1480. https://doi.org/10.1021/bm060010d.
- [71] K.Y. Lee, D.J. Mooney, Alginate: Properties and biomedical applications, Prog. Polym. Sci. 37 (2012) 106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
- [72] S.K. Bajpai, S.K. Saxena, S. Sharma, Swelling behavior of barium ions-crosslinked bipolymeric sodium alginate – carboxymethyl guar gum blend beads, React. Funct. Polym. 66 (2006) 659–666. https://doi.org/10.1016/j.reactfunctpolym.2005.10.019.
- [73] A.C.K. Bierhalz, A. Mariana, M.E.M. Braga, H.J.C. Sousa, T.G. Kieckbusch, LWT - Food Science and Technology Effect of calcium and / or barium crosslinking on the physical and antimicrobial properties of natamycin-loaded alginate fi lms, LWT - Food Sci. Technol. 57 (2014) 494–501. https://doi.org/10.1016/j.lwt.2014.02.021.
- [74] V.F. Duvivier-Kali, A. Omer, R.J. Parent, J.J. O’Neil, G.C. Weir, Complete Protection of Islets Against Allorejection and Autoimmunity by a Simple Barium-Alginate Membrane, Diabetes. 50 (2001) 1698–1705. https://doi.org/10.2337/diabetes.50.8.1698.
- [75] P. Gröhn, G. Klöck, J. Schmitt, U. Zimmermann, A. Horcher, R.G. Bretzel, B.J. Hering, D. Brandhorst, H. Brandhorst, T. Zekorn, K. Federlin, Large-scale production of Ba2+-alginate-coated islets of Langerhans for immunoisolation, Exp. Clin. Endocrinol. Diabetes. 102 (1994) 380–387. https://doi.org/10.1055/s-0029-1211308.
- [76] P. Gröhn, G. Klöck, U. Zimmermann, Collagen-coated Ba2+-alginate microcarriers for the culture of anchorage-dependent mammalian cells, Biotechniques. 22 (1997) 970–975. https://doi.org/10.2144/97225rr06.
- [77] C.J. Duan, X.Y. Wu, W. Liu, H.H. Chen, X.X. Yang, J.T. Zhao, X-ray excited luminescent properties of apatitic compounds Ba 5(PO4)3X (X: OH-, Cl-, Br-); Structure and hydroxyl ion conductivity of barium hydroxylapatite, J. Alloys Compd. 396 (2005) 86–91. https://doi.org/10.1016/j.jallcom.2004.11.064.
- [78] C.H. Yoder, J.D. Pasteris, K.A. Krol, V.L. Weidner, R.W. Schaeffer, Synthesis, structure, and solubility of carbonated barium chlor- and hydroxylapatites, Polyhedron. 44 (2012) 143–149. https://doi.org/10.1016/j.poly.2012.06.039.
- [79] A. Yasukawa, E. Ueda, K. Kandori, T. Ishikawa, Preparation and characterization of carbonated barium-calcium hydroxyapatite solid solutions, J. Colloid Interface Sci. 288 (2005) 468–474. https://doi.org/10.1016/j.jcis.2005.03.007.
- [80] H. Liu, Z. Zhang, C. Gao, Y. Bai, B. Liu, W. Wang, Y. Ma, Saijilafu, H. Yang, Y. Li, A. Chan, L. Yang, Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement, Mater. Sci. Eng. C. 116 (2020) 110904. https://doi.org/10.1016/j.msec.2020.110904.
- [81] N.J. Flora, K.W. Hamilton, R.W. Schaeffer, C.H. Yoder, A Comparative study of the synthesis of calcium, strontium, barium, cadmium, and lead apatites in aqueous solution, Synth. React. Inorg. Met. Chem. 34 (2004) 503–521. https://doi.org/10.1081/SIM-120030437.
- [82] A. Bigi, E. Foresti, F. Marchetti, A. Ripamonti, N. Roveri, Barium calcium hydroxyapatite solid solutions, J. Chem. Soc. Dalt. Trans. 5 (1984) 1091–1093. https://doi.org/10.1039/DT9840001091.
- [83] M. Yashima, Y. Kawaike, Crystal Structure and Site Preference of Ba-Doped r -Tricalcium Phosphate ( Ca 1 - x Ba x ) 3 ( PO 4 ) 2 through High-Resolution Synchrotron Powder Diffraction ( x ) 0 . 05 to 0 . 15 ), Chem. Mater. 3 (2007) 3973–3979.
- [84] M. Myat-htun, A.M. Noor, M. Kawashita, Enhanced sinterability and in vitro bioactivity of barium-doped akermanite ceramic, Ceram. Int. 46 (2020) 19062–19068. https://doi.org/10.1016/j.ceramint.2020.04.238.
- [85] S.K. Arepalli, H. Tripathi, V.K. Vyas, S. Jain, S.K. Suman, R. Pyare, S.P. Singh, Influence of barium substitution on bioactivity, thermal and physico-mechanical properties of bioactive glass, Mater. Sci. Eng. C. 49 (2015) 549–559. https://doi.org/10.1016/j.msec.2015.01.049.
- [86] O. Acarturk, M. Lehmicke, H. Aberman, D. Toms, J.O. Hollinger, M. Fulmer, Bone Healing Response to an Injectable Calcium Phosphate Cement With Enhanced Radiopacity, J. Biomed. Mater. Res. Part B Appl. Biomater. (2007) 56–62. https://doi.org/10.1002/jbm.b.30987.
- [87] S.K. Bajpai, S. Sharma, Investigation of pH ‐ Sensitive Swelling and Drug Release Behavior of Barium Alginate / Carboxymethyl Guar Gum Hydrogel Beads Investigation of pH-Sensitive Swelling and Drug Release B, J. Macromol. Sci. , Part A Pure Appl. Chem. 43 (2006) 1513–1521. https://doi.org/10.1080/10601320600896728.
- [88] Y.A. Mørch, M. Qi, P.O.M. Gundersen, K. Formo, I. Lacik, J. Oberholzer, B.L. Strand, Binding and leakage of barium in alginate microbeads, (2012) 2939–2947. https://doi.org/10.1002/jbm.a.34237.
- [89] B.Y.E.M. Gallantt, BARIUM-TREATED MAMMALIAN SKELETAL MUSCLE: SIMILARITIES TO HYPOKALAEMIC PERIODIC PARALYSIS, J. Physiol. (1983) 577–590.
- [90] W. Walz, M. Shargool, L. Hertz, Barium-induced inhibition of K+ transport mechanisms in cortical astrocytes-its possible contribution to the large Ba2+-evoked extracellular K+ signal in brain, Neuroscience. 13 (1984) 945–949. https://doi.org/10.1016/0306-4522(84)90108-8.
- [91] L. Mores, E.L. França, N.A. Silva, E.A. Suchara, A.C.H.F. França, Nanoparticles of barium induce apoptosis in human phagocytes, Int. J. Nanomedicine. (2015) 6021–6026.
