RNAi technology is a versatile, effective, safe, and eco-friendly alternative for crop protection. There is plenty of evidence of its use through host-induced gene silencing (HIGS) and emerging evidence that spray-induced gene silencing (SIGS) techniques can work as well to control viruses, bacteria, fungi, insects, and nematodes. For SIGS, its most significant challenge is achieving stability and avoiding premature degradation of RNAi in the environment or during its absorption by the target organism.
- RNAi
- encapsulation
1. Introduction
The world is moving toward a more sustainable crop production system that requires specific and efficient tools to battle plant pathogens. RNAi can be used for such purposes. The molecule is used in nature, degrades quickly, can disrupt the pathogen at a genetically specific level, and can complement the current agronomic crop protection practices used for organic, conventional, ecological, or technological production [1]. The reader may be familiar with the concept of DNA and genes located in the nucleus of eukaryote cells, containing the instructions to create organic molecules, mainly proteins. RNA messenger works as an intermediator, carrying the nucleus’s message to the cytoplasm to be read by the ribosomes to assemble the protein. The RNAi eukaryotic machinery is a complex system for virus defense and gene expression control, sometimes called post transcriptional gene silencing (PTGS). The system can be triggered by external specific dsRNA, resulting in its RNA messenger being blocked before it gets to the ribosome, leaving the organism, such as a pathogen, disarmed [2]. The extravesical delivery of dsRNA to disarm the expression system was proven to be natural and bidirectional from plant to fungal pathogens and vice versa cross-kingdom communication [3,4,5,6,7,8].
Consequently, RNAi represents an opportunity to emulate or improve the natural plant pathogen control system by providing well-designed external dsRNA [9]. Here, we aimed to present advantages in crop protection mediated by RNAi. There are two RNAi plant-based technologies: host-induced gene silencing (HIGS) since the 1990s and emerging spray-induced gene silencing (SIGS). Both can provide sustainable solutions to control pathogens, such as insects, viruses, and fungi. We will focus on SIGS because it is becoming an emerging affordable option with a cost reduction of approximately USD 0.5–1 per gram [10]; the small amount of dsRNA necessary seems to be near 2–10 g per hectare; it is safe; and it has fast environmental degradation [11,12,13]. When dsRNA is applied externally to plants, one proposed mechanism is that plant cells can take it and use it directly to tackle the pathogen through secreted vesicles containing the RNA at the site of the infection and plasmodesmata [14,15,16]. The amount of sprayed RNA may vary depending on the target species’ sensitivity to RNAi, the capacity to trigger the defense system, and the efficiency of the delivery method. Other challenges for this technology are the need for science-based risk assessment procedures for topical RNAi applications within existing plant protection product legislation, regulatory approaches [12,13], and strategies to use more than one target sequence to avoid resistance of uptake [17].
2. How it Works
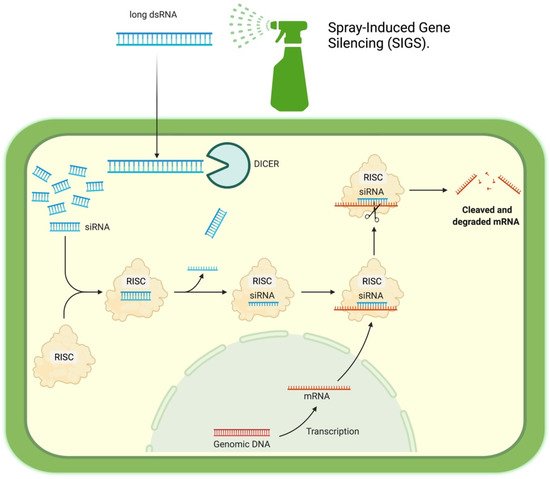
3. Potential Targets
The potential targets of RNAi can be viruses, fungi, bacteria, nematodes, and endogenous genes. Next, we describe a general table ( Table 1 ) containing several targets to demonstrate that the technology is flexible enough to start exploring other plagues, and broader reviews exist in case of interest by the reader [23]. Out of these possibilities, we would like to remark on the potential of using SIGS to produce a new generation of crop protection-specific products.
Table 1. Potential targets for spray-induced gene silencing (SIGS) in plants.
| Target | Experimental Evidence | Target Genes | Reference |
|---|---|---|---|
| Virus | dsRNA + clay resulted in BCMV virus resistance for 20 d | Nib and CP genes of BCMV | [24] |
| TMV Tobacco virus resistance for 7–20 d | CP, P126, RP of TMV | [25,26] | |
| Fungi | Inhibits Botrytis cinerea disease | DCL1, DCL2 of Botrytis cinerea | [27] |
| Efficiently inhibited Fusarium graminearum | CYP51A, CYP51B, CYP51C of F. graminearum | [28] | |
| Sclerotinia sclerotiorum/Botrytis cinerea | mRNA splicing, ribosome biogenesis, protein disulfide oxidoreductase, peroxisomal protein | [27] | |
| Fusarium asiaticum, Botrytis cinerea, Magnaporthe oryzae, Colletotrichum truncatum | β2-tubulin | [29] | |
| Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensi, Fusarium | adenylate cyclase, DNA polymerase alpha subunit/delta subunit/CYP51 | [30,31] | |
| Nematodes | Caenorhabditis elegans, Radopholus similis, Meloidogyne artiellia, Meloidogyne incognita, Globodera pallida | Several genes such as 16D10 peptide, chitin synthase, xylanase, glutathione-S- transferase, FMRF-like peptides | [18,32,33,34,35,36] |
| Insects | Coleopterans are highly sensitive, Hemiptera, Orthoptera, Diptera, Hymenoptera, and Lepidoptera have different responses. | Several genes such as salivary protein C002, 16D10, αCOP, Cytochrome P450, Acetylcholinesterase, ABC transporter, β-actin, chitin synthase B | [37,38,39,40,41,42] |
| Endogenous plant genes | Arabidopsis, Tobacco, poplar, rice | Transgenes/CHS/EPSPS/STM/WER/MYB1/WRKY23 | [43] |
4. Encapsulation Technology to Improve Efficiency
| Encapsulation System | Potential Crop Protection Application |
Strategy | Reference |
|---|---|---|---|
| Guanylated 2-(aminoethyl) methacrylate (AEMA)/dsRNA polyplex nanoparticles. |
Insecticide induces decreased feeding in Lepidopteran larvae (Spodoptera exigua), then promoting weight loss, developmental halt, and mortality. | Increases RNAi efficiency in targeting the essential gene chitin synthase B (ChSB), while preventing the degradation of dsRNA in the alkaline gut of insects and enhancing its cellular uptake in midgut cells. | [47] |
| poly-[N-(3-guanidinopropyl) methacrylamide] (pGPMA)/dsRNA interpolyelectrolyte nanocomplex. | Ingestion insecticide regulates gene silencing in Lepidopteran larvae (Spodoptera frugiperda), increasing mortality from starvation and growth stunting. | Increased internalization and protection of dsRNA in insect cells, decreasing the accumulation of target mRNA due to the knockdown of genes related to vital functions such as nutrient absorption (sfVATPase), intracellular transport (sfKIF), and cell division (sfCDC27). | [52] |
| Chitosan/dsRNA polyplex nanoparticles |
Nematicide can homogeneously enter the nematode’s body (Caenorhabditis elegans) through noncanonical endocytotic pathways and attack specific genes. The combined effect decreases the development of the nematode by the action of the chitosan vehicle. | Increases RNAi efficiency of gene knockdown throughout the whole body of the nematode by introducing intact dsRNA through the Clathrin-mediated endocytosis pathway, which is different from the canonical pathway (sid-1 and sid-2) in the study model. Furthermore, chitosan was shown to effectively decrease myosin gene expression, which is critical for the growth and reproduction of the model nematode. | [22] |
| Chitosan/dsRNA polyplex nanoparticles |
Insecticide against Lepidopteran larvae (Spodoptera frugiperda) acts on genes related to the apoptosis pathway, inducing growth impairment and larval mortality. | Improves RNAi efficiency through the protection of dsRNA from degradation by intracellular and intercellular RNases. It also reduces the accumulation of dsRNA in the endosome while favoring its transport to the cytoplasm, where the formation of siRNAs is promoted, producing knockdown of apoptosis-related genes (iap). | [45] |
| Layered double hydroxide (LDH) clay nanosheets/dsRNA | Develop a topical product that induces viral resistance in plants (against PMMoV and CMV) using dsRNA absorption technology in clay nanosheets (Bio-Clay). | Increased persistence of the topical treatment due to strong adhesion of dsRNA in the vehicle (LDH) and with the leaves. It also allows the controlled release of the biomolecule and confers protection against environmental degradation while favoring the internalization of dsRNA in the plant. | [44] |
| Lipofectamine 2000 liposomes/dsRNA. | Insecticide against Diptera of the genus Drosophila (D. melanogaster, D. sechellia, D. yakuba, and D. pseudoobscura) acting by ingestion. It attacks essential genes of development through knockdown management. | Promotion of dsRNA internalization in insects through encapsulation protection, increasing silencing efficiency by promoting more significant RNAi accumulation in larvae. Knockdown of the genes of the VATPase (gut lumen pH stabilizer associated with nutrient uptake) and gTub23C (mitosis-related g-tubulin protein, essential for microtubule organization). | [55] |
| Lipofectamine 2000 liposomes/dsRNA. | Specific insecticide against larvae and adults of Drosophila suzukii combining synergic effect of multiple gene knockdown. Oral administration route. | It facilitates uptake in the insect’s gut. It causes significant mortality in larvae and adults by the reduction in transcript levels of essential genes rps13 (housekeeping), alpha COP (coatomer subunit for trans-organelles transport), and vha26 (subunit of the vacuolar ATPase). The synergistic action of knockdown of the rps13 and alpha COP genes significantly increases mortality in the insect. | [53] |
| Liposomes/dsRNA | Oral insecticide for the control of nymphs of Euschistus heros (hemiptera: pentatomidae), which is one of the main soybean pests in the field. | Protection of dsRNA against degradation promoted by the ribonuclease action of insect saliva. Enhanced silencing activity of target genes vATPaseA (V-type proton ATPase catalytic subunit A) and act-2 (muscle actin). | [56] |
| Recombinant Flock House Virus FHV/dsRNA | Recombinant insecticide based on a viral vehicle transporting dsRNA silencers of essential genes in Drosophila melanogaster. For potential massive application in other species susceptible to FHV infection. | Use of the insect cell machinery to assemble infective recombinant FHV virions that carry target sequences for the production of dsRNA when replicating in cells. Thus, virions protect the sequences responsible for silencing the rps13 (housekeeping), alpha COP (coatomer subunit for trans-organelle transport), and vha26 (subunit of the vacuolar ATPase) genes while at the same time favoring dispersal in insects. It simulates natural viral infection. | [54] |
| Virus-Like Particles (VLP)/dsRNA | Oral insecticide for the control of ants of several genera (Solenopsis invicta (fire ants), Camponotus pennsylvanicus and Camponotus floridanus (carpenter ants), Linepithema humile (Argentine ants), Tapinoma sessile (odorous ants), Tetramorium caespitum (pavementom ants), and Monstrous ants) pharaonis (pharaoh ants); inducing the silencing of physiological genes required for the survival of the colony. | Recombinant production in E. coli, which through specific plasmids manufactures capsid proteins of bacteriophages Qß and MS2 and inducible RNAi precursor sequences. The packaging of dsRNAs in VLPs protects them from degradation by nonspecific environmental organisms and the intestinal RNases of the target organism. It also favors its absorption by lining the gut cells. The silenced genes are related to the viability of the colony, for example, the induction of sterility and individual mortality. VLP carrying dsRNA is sprayed on the ground for spot application or incorporated into the bait. Target genes include VgR (vitellogenin receptor protein), TVXl (telomerase variant XI protein), PBAN (pheromone biosynthesis activating neuropeptide), PBANR (pheromone biosynthetic activating neuropeptide receptor), WLS (wntless protein), MEGF10 (multiple epidermal growth factor-like domain proteins 10), CHCP (clatherin heavy chain protein), CDC7 (cell division cycle 7-related protein), Cep89 (centrosomal protein 89 kdal), PSMBl (beta subunit of the type-1 proteosome), A5C (actin 5C protein), ATPSD (ATP synthase delta subunit); as well others related with anamorsin, beta actin, and Csp9 proteins | [46] WO2017/136353Al for APSE RNA Containers (ARCs) |
| Ribonucleoprotein particle (RNP)/dsRNA | Insecticide for control of cotton boll weevil (Anthonomus grandis) adults. | Developing a protection and stability system for dsRNA avoids degradation by nucleases in the insect’s gut and favors rapid cellular incorporation. The above is based on a chimeric protein PTD-DRBD (peptide transduction domain–dsRNA binding domain) combined with dsRNA. This type of resulting protein is known as cell-penetrating peptide (CPP). | [51] |
5. Conclusions and Future Perspectives
RNAi technology is a powerful and versatile alternative for pest and disease control in crops. Its use in the agricultural field extends to viruses, bacteria, fungi, insects, nematodes, and plants. It grows steadily with other complementary technologies, such as the recombinant production of RNAi in vectors, transgenesis, and micro/nanoencapsulation of candidate si/dsRNA. The main issue preventing its adoption in the past was the cost of production and stability. The cost of production is getting lower with the development of new technologies, while stability encapsulation strategies provide a solution to avoid degradation.
Together with the positive approaches to regulation, the emergence of more interdisciplinary alternatives that combine gene silencing by RNAi is also expected. For instance, the induction of resistance in crops by elicitation and metabolic control methods, using the strengths of both. Following this approach, we are developing a technology that uses elicitor nanoparticles made of natural polymers to induce defense in plants, which will also carry a double control mechanism based on RNAi to unblock inhibitors of systemic defense against systemic defense pathogenic species of the genus Fusarium . There is also interest in the scientific community to produce multiple knockdowns that protect systemically against a consortium of pathogens under the same application. Another challenge for this technology is to keep reducing production costs, for which biotechnology is emerging as one of the main allies to improve profitably on a large scale.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222212148
