Wood- or plant-based cellulose fibres have shown their potential as a reinforcement in composites for a relatively long time alongside the commonly used glass-fibre and carbon-fibre reinforcements. Whereas regular biocomposites suffer from fibre-matrix adhesion-related challenges, all-cellulose composites (ACCs) can overcome this problem by both matrix and reinforcement having the same or a similar chemical structure, which results in good interfacial compatibility. ACCs can provide an environmentally friendly alternative to conventional petrochemical-based materials since they are a type of single-polymer composites (SPCs) from biomass-derived cellulose, and as such, they are easily recyclable, and they originate from renewable sources.
- all-cellulose composite
1. Cellulose
1.1. Characteristics of Cellulose
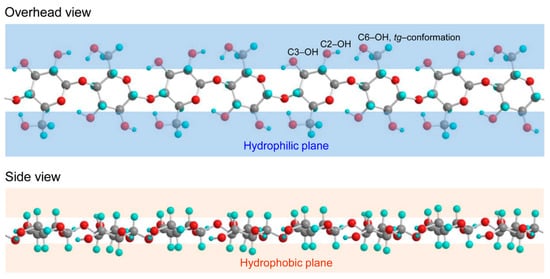
1.2. Nanocellulose
| NAME | ABBREVIATION | WIDTH | LENGTH | L/D RATIO |
|---|---|---|---|---|
| Cellulose nanocrystal | CNC (NCC) | (I) 3–10 nm (II) 3–20 nm |
(II) 50–500 nm | (I) > 5 |
| Cellulose nanofibril | CNF (NFC) | (I) 5–30 nm (II) 3–100 nm |
(II) 0.5–2 µm | (I) > 50 |
| Cellulose microcrystal | CMC (MCC) | (I) 10–15 µm (II) 10–50 µm |
(II) 10–50 µm | (I) < 2 |
| Cellulose microfibril | CMF (MFC) | (I) 10–100 nm (II) 10–100 nm |
(I) 0.5–50 µm (II) 0.5–10’s µm |
2. All-Cellulose Composites
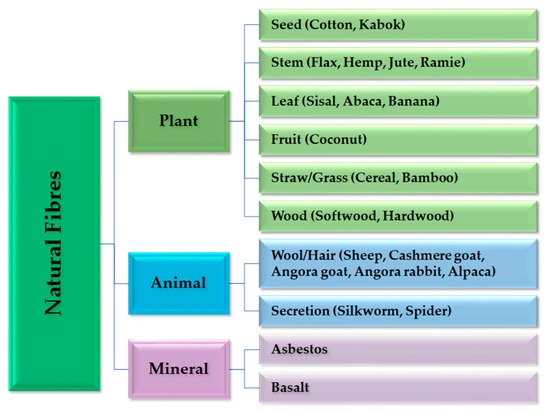
2.1. Raw Materials for the ACC
2.2. Biocomposites
2.3. Matrix-Reinforcement Compatibility
This entry is adapted from the peer-reviewed paper 10.3390/app112110069
References
- Bos, H.L.; Oever, V.M.J.A.; Peters, O.C.J.J. Tensile and compressive properties of flax fibres for natural fibre reinforced composites. J. Mater. Sci. 2002, 37, 1683–1692.
- Sabater, M.J.; Ródenas, T.; Heredia, A. Biopolymers from Plants. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 37–86.
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393.
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81.
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in)solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587.
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148.
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose-structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21.
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55.
- Winkworth-Smith, C.; Foster, T.J. General overview of biopolymers: Structure, properties, and applications. In Handbook of Biopolymeric Materials; Thomas, S., Durand, D., Chassenieux, C., Jyotishkumar, P., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013.
- Olsson, C.; Westman, G. Direct Dissolution of Cellulose: Background, Means and Applications. In Cellulose-Fundamental Aspects; Van de Ven, T., Godbout, L., Eds.; IntechOpen: London, UK, 2013.
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 1, pp. 1–49.
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40.
- Cai, C.; Antikainen, J.; Luostarinen, K.; Mononen, K.; Heräjärvi, H. Wetting-induced changes on the surface of thermally modified Scots pine and Norway spruce wood. Wood Sci. Technol. 2018, 52, 1181–1193.
- Belgacem, M.N.; Gandini, A. Production, chemistry and properties of cellulose-based materials. In Biopolymers-New Materials for Sustainable Films and Coatings; Plackett, D., Ed.; Wiley: West Sussex, UK, 2011; pp. 151–178.
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials. De Gruyter: Berlin, Germany, 2012.
- Ayoub, A.; Lucia, L.A. Introduction to Renewable Biomaterials: First Principles and Concepts; John Wiley & Sons: Hoboken, NJ, USA, 2018.
- Zugenmaier, P. Crystalline Cellulose and Derivatives: Characterization and Structures, Springer Series in Wood Science; Springer: Berlin, Germany, 2008; pp. 175–206.
- Kumar, A.; Negi, Y.S. Cellulose Nanocrystals: Nanostrength for Industrial and Biomedical Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 393–435.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Pandey, J.K.; Nakagaito, A.N.; Takagi, H. Fabrication and applications of cellulose nanoparticle-based polymer composites. Polym. Eng. Sci. 2013, 53, 1–8.
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. Appl. Polym. Sci. 1983, 37, 815–827.
- Nakagaito, A.N.; Yano, H. Cellulose-Nanofiber-Based Materials. Cellul. Based Compos. New Green Nanomater. 2014, 1, 1–25.
- Hietala, M.; Oksman, K. Technologies for Separation of Cellulose Nanofibers. In Handbook of Green Materials; World Scientific: Singapore, 2014; pp. 53–71.
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 115–141.
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989.
- Filipova, I.; Serra, F.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Oxidative treatments for cellulose nanofibers production: A comparative study between TEMPO-mediated and ammonium persulfate oxidation. Cellulose 2020, 27, 10671–10688.
- Wågberg LDecher GNorgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The Build-Up of Polyelectrolyte Multilayers of Microfibrillated Cellulose and Cationic Polyelectrolytes. Langmuir 2008, 24, 784–795.
- Sirviö, J.A. Cationization of lignocellulosic fibers with betaine in deep eutectic solvent: Facile route to charge stabilized cellulose and wood nanofibers. Carbohydr. Polym. 2018, 198, 34–40.
- de Souza Lima, M.M.; Borsali, R. Rodlike Cellulose Microcrystals: Structure, Properties, and Applications. Macromol. Rapid Commun. 2004, 25, 771–787.
- Eichhorn, S.J. Characterization of Cellulose Nanofiber-based Nanocomposite Interfaces. In Handbook of Green Materials; World Scientific: Hackensack, NJ, USA, 2014; pp. 107–120.
- Zadorecki, P.; Michell, A.J. Future prospects for wood cellulose as reinforcement in organic polymer composites. Polym. Compos. 1989, 10, 69–77.
- Oksman Niska, K.; Mathew, A.P.; Bismarck, A.; Rojas, O.J.; Sain, M. Handbook of Green Materials: Processing Technologies, Properties and Applications; World Scientific: Hackensack, NJ, USA, 2014.
- Huang, S.; Liu, X.; Chang, C.; Wang, Y. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011.
- Hult, E.; Larsson, P.T.; Iversen, T. Cellulose fibril aggregation—An inherent property of kraft pulps. Polymer 2001, 42, 3309–3314.
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 2009, 17, 19–30.
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–35.
- Mokhothu, T.H.; John, M.J. Review on hygroscopic aging of cellulose fibres and their biocomposites. Carbohydr. Polym. 2015, 131, 337–354.
- Takagi, H. Fabrication and Evaluation of Cellulose-Nanofiber-Reinforced Green Composites. In Cellulose Based Composites: New Green Nanomaterials; Hinestroza, J.P., Netravali, A.N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 27–42.
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43.
- Chen, C.; Duan, C.; Li, J.; Liu, Y.; Ma, X.; Zheng, L.; Stavik, J.; Ni, Y. Cellulose (Dissolving Pulp) Manufacturing Processes and Properties: A Mini-Review. BioResources 2016, 11, 5553–5564.
- Björquist, S.; Aronsson, J.; Henriksson, G.; Persson, A. Textile qualities of regenerated cellulose fibers from cotton waste pulp. Text. Res. J. 2018, 88, 2485–2492.
- Renewcell Technology. 2021. Available online: https://www.renewcell.com/en/section/our-technology (accessed on 20 May 2021).
- Ganster, J.; Fink, H.; Uihlein, K.; Zimmerer, B. Cellulose man-made fibre reinforced polypropylene—correlations between fibre and composite properties. Cellulose 2008, 15, 561–569.
- Tan, P.; Tong, L.; Steven, G.P. Modelling for predicting the mechanical properties of textile composites—A review. Compos. Part A Appl. Sci. Manuf. 1997, 28, 903–922.
- Jones, I.A.; Pickett, A.K. Mechanical properties of textile composites. In Design and Manufacture of Textile Composites; Long, A.C., Ed.; Woodhead Publishing: Sawston, UK, 2005; pp. 292–329.
- Kabir, S.M.F.; Chakraborty, S.; Hoque, S.M.A.; Mathur, K. Sustainability Assessment of Cotton-Based Textile Wet Processing. Clean Technol. 2019, 1, 16.
- Mathur, K. New Developments in Fibers, Yarns & Fabrics. Text. World 2020, 170, 32–35.
- Ecovero; The New Standard in Eco-Responsible. 2021. Available online: https://www.ecovero.com (accessed on 20 May 2021).
- Lenzing; Tencel, T.M. 2021. Available online: https://www.lenzing.com/products/tenceltm. (accessed on 20 May 2021).
- Kuura; Metsä Group’s New Textile Fibre is Kuura. 2021. Available online: https://www.kuura.io/metsa-groups-new-textile-fibre-is-kuura (accessed on 1 June 2021).
- Infinited Fiber. It’s Time for the Textile Industry to Lose Its Virginity. 2021. Available online: https://infinitedfiber.com (accessed on 20 May 2021).
- Haksa Textile Environmentally Friendly Yarn. 2021. Available online: http://haksatekstil.com.tr/en/home-page (accessed on 20 May 2021).
- Spinnova Technology. 2021. Available online: https://spinnova.com/technology (accessed on 20 May 2021).
- Temmink, R.; Baghaei, B.; Skrifvars, M. Development of biocomposites from denim waste and thermoset bio-resins for structural applications. Compos. Part A Appl. Sci. Manuf. 2018, 106, 59–69.
- Baghaei, B.; Compiet, S.; Skrifvars, M. Mechanical properties of all-cellulose composites from end-of-life textiles. J. Polym. Res. 2020, 27, 1–9.
- Chinga-Carrasco, G. (Ed.) Novel Biocomposite Engineering and Bio-Applications; MDP-Multidisciplinary Digital Publishing institute: Basel, Switzerland, 2019.
- Müssig, J.; Stevens, C. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2010.
- Kumar, T.V.; Singh, S.A. Natural Plant Fibre Biocomposites for Structural Vehicle Components. In Biomass-Based Biocomposites; Smithers Rapra Technology: Shawbury, UK, 2013.
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26.
- Väisänen, S.; Ajdary, R.; Altgen, M.; Nieminen, K.; Kesari, K.K.; Ruokolainen, J.; Rojas, O.J.; Vuorinen, T. Cellulose dissolution in aqueous NaOH–ZnO: Cellulose reactivity and the role of ZnO. Cellulose 2021, 28, 1267–1281.
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364.
- Sorieul, M.; Dickson, A.; Hill, S.; Pearson, H. Plant Fibre: Molecular Structure and Biomechanical Properties, of a Complex Living Material, Influencing Its Deconstruction towards a Biobased Composite. Materials 2016, 9, 618.
- Lee, K.Y.; Bismarck, A. Green Chemical Modifications of Nanocellulose for Use in Composites. Handb. Green Mater. 2014, 2, 7–21.
- Li, J.; Nawaz, H.; Wu, J.; Zhang, J.; Wan, J.; Mi, Q.; Yu, J.; Zhang, J. All-cellulose composites based on the self-reinforced effect. Compos. Commun. 2018, 9, 42–53.
- Campbell, F.C. Structural Composite Materials; ASM International: Almere, The Netherlands, 2010.
- Thakur, V.K.; Thakur, M.K.; Kessler, M.R. Handbook of Composites from Renewable Materials. Physico-Chemical and Mechanical Characterization; Scrivener Publishing: Austin, TX, USA, 2017; Volume 3.
- Chawla, K.K. Composite Materials: Science and Engineering; Springer: New York, NY, USA, 2013.
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecule 2020, 25, 2836.
- Alcock, B.; Cabrera, N.O.; Barkoula, N.-; Loos, J.; Peijs, T. The mechanical properties of unidirectional all-polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 716–726.
- Retegi Miner, A.; Zuluaga Gallego, R.; Gañán Rojo, P.; Mondragon, I. Nanocomposites Based on Matrices Extracted from Vegetable Oils and Bacterial Cellulose. In Cellulose Based Composites: New Green Nanomaterials; Hinestroza, J.P., Netravali, A.N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 63–78.
- Capiati, N.J.; Porter, R.S. The concept of one polymer composites modelled with high density polyethylene. J. Mater. Sci. 1975, 10, 1671–1677.
- Phillips, H.M. Vulcanized Fibre. U.S. Patent 3,189,515, 15 June 1965.
- Brown, W.F. Vulcanized fibre–an old material with a new relevancy. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference, Cincinnati, OH, USA, 28 October 1999; pp. 309–312.
