The abscopal effect (AbE) is defined as radiation-induced shrinkage of distant, non-treated, neoplastic lesions and it is considered the best clinical picture of the efficient immune stimulation by irradiation.
- abscopal effect
- HNSCC
- precision medicine
1. Introduction
Head and neck squamous cell cancer (HNSCC) is the sixth most common cancer worldwide, more than 90% involving oral cavity, pharynx, or larynx. Known risk factors are excessive use of tobacco and alcohol, acquiring infection with human papilloma virus, especially HPV 16, HPV 18, and Epstein–Barr virus [1]. Radiotherapy represents a cornerstone in its management, and it is clinically implemented in different regimens; for locally advanced disease, modulated intensity radiotherapy (IMRT) is classically used, with daily doses ranging from 1.8 to 2.0 Gy, either alone or in combination with concomitant chemotherapy or cetuximab [2][3]. In recurrent or metastatic disease, the standard first line is platinum-based chemotherapy with 5-fluorouracil and cetuximab [4], however, stereotactic body radiotherapy (SBRT) with fractionation between 8 and 15 Gy is widely used [5]; in fact, thanks to its high spatial precision it could achieve a better control of local symptoms reducing toxicities. Its growing use increased reports of the abscopal effect as a systemic distant response of non-irradiated tumors or metastasis, as a consequence of proinflammatory changes in tumor microenvironment (TME) against cancer antigens [6][7]. A further boost has been given by the introduction of the immunotherapy targeting Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) and Programmed Death–Ligand 1 (PD-L1)/PD-1 axis: RT stimulates a robust tumor antigen cross-presentation in nodes while CTLA-4 blockade enhanced the priming of responsive T-cells in TME, highlighting an interesting clinical synergism. [8][9]. The goal of radioimmunotherapy should be to stimulate but also improve the duration of the immune response. Among various combination strategies, SBRT is most effective in inducing the abscopal effect, although the right dose and ideal fractionation has yet to be identified [9].
2. Insight into the Abscopal Effect: Modulation of the Tumor Microenvironment (TME) by Irradiation
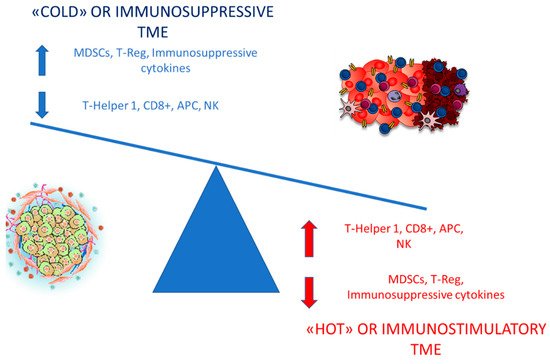
3. Radiotherapy and Immune-Checkpoint Inhibitors (ICIs): Potentially a Well Balanced Synergism
Radiotherapy immunogenicity is strictly related to tumor-specific characteristics, such as heterogeneity and radiosensitivity, and specific RT aspects like dose, fractionation, and timing of administration. Most of the clinical data regarding the use of radio-immunotherapy combinations are limited to either anti-CTLA-4 or anti-PD-1 agents: RT stimulates antigen cross-presentation and T-cell priming in draining nodes while CTLA-4 inhibitors enhance priming phase of effector T-cell activation induced by RT, leading to pro-immunogenic infiltrate of the TME. Blocking CTLA-4 enhances T-cell activation, increasing the CD8+/T-reg ratio, and strengthening the in situ vaccination effect [35][36][37][38][39].
PD-1 is expressed on T-cells, DCs, and NK cells: PD-1/PD-L1 pathway primarily inhibits T-cell proliferation by blocking cell-cycle progression, thus protecting tumor cells from T-cell attack. A recent study suggested that radiation-induced double-strand breakage of DNA results in upregulation of the expression of PD-L1 thanks to Ataxia-telangiectasia-mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) kinases [40][41][42][43]. Therefore, PD-L1 hyperexpression on cancer cells could be associated with a higher rate of responses to specific inhibitors while radiotherapy could act as a “Trojan horse” [42][43].
An excellent murine model published by Twyman-Saint Victor et al. clarified that radiotherapy and anti-CTLA4 monoclonal antibodies (mAbs) led to upregulation of PD-L1 on melanoma cells, mediating T-cell exhaustion and explaining at least in part the limited local and abscopal responses observed [44]. Anti-CTLA4 predominantly inhibits T-reg cells, thereby increasing CD8/T-reg ratio while radiation enhances the diversity of T-cell receptor (TCR) repertoire of intratumoral T-cells [44]. Addition of PD-L1 inhibitor reverses T-cell exhaustion, mitigates depression in the CD8/Treg ratio, and further encourages oligoclonal T-cell expansion [44]. Similarly to results from mice, patients with melanoma showing high PD-L1 levels on neoplastic cells did not respond to radiotherapy plus anti-CTLA4 mAb, demonstrated persistent T-cell exhaustion and rapidly progressive disease.
Evidence regarding the optimal timing, dose, schedules, sequences, and fractionation of radiotherapy are conflicting. Pre-clinical studies highlighted that starting anti-PD-L1 treatment 7 days following RT was inferior to starting on either the first or the last day [45]; however, some data also show how radiotherapy-anti PD-L1 sequence, with late administration of the checkpoint inhibitor, reinvigorates exhausted T-cells while an early sequence favors the differentiation and initial activation of T-cells [46][47]. Nowadays, it is clear that there is a synergism between immunotherapy and RT either as a single-fraction or in fractionated courses. Following irradiation with 12 Gy on 2 consecutive days, overall leukocyte and CD8+ T-cell frequencies peak at 5 days post-RT and then gradually decline to pre-RT levels [48]. Five days post-RT also reflects the highest CD8/T-Reg ratio, probably the ideal time-point for checkpoint blockade. Moreover, Frey et al. showed that after irradiation of 5 Gy × 2 fractions, CD8+ peak at day 8 and then decline while T-regs have a bimodal peak on days 8 and 10 [49]. Knowing the kinetics of infiltration of immune cells should be correlated with the time of administration of the ICIs, in order to achieve the maximum immunostimulant effect.
Hypofractionated radiotherapy (hRT) is the delivery of fewer, larger (>2 Gy) doses of radiotherapy and is a potential strategy for improving dose intensity. hRT appears particularly immunogenic: T lymphocytes, including Tumor-infiltrating lymphocytes (TILs), have generally been considered as highly radiosensitive [50][51], that is why it could be postulated that extending hRT schedules might be less immunogenic if administered in the period in which T lymphocytes, stimulated by RT/ICI combination, migrate to TME [50][51]. Following the experience of Frey [49], Zhang et al. compared a combination of anti-PD1 treatment and hRT with different schedules and equivalent biologically effective doses in mice affected by melanoma. Anti-PD1 antibody was given weekly while primary tumor was irradiated with 3 × 9.18 Gy in 3 or 5 days or with 5 × 6.43 Gy in 10 days [52]. All the combinations inhibited growth of irradiated primary and non-irradiated secondary tumors greater than hRT and anti-PD1 monotherapy; similarly, local and systemic tumor-specific CD8+ T-cell responses and TILs were also similar across short or extended hRT schedules [52]. Zhang’s experience also highlighted how regional and abscopal antineoplastic biological effects of the extended schedules are similar to the shorter ones only if the regional lymph nodes provide sufficient cancer-specific T-cells in the TME [52].
Historically, HNSCC was considered poorly responsive to high-dose hRT rather than low-dose hRT, however, low-dose schedules may induce lymphopenia and immunosuppression [53]. Morisada et al. analyzed immune correlates, primary tumor, and abscopal control rates as a result of two different radio-immunotherapy combinations of PD-1 monoclonal antibody with either daily low-dose fractionated radiotherapy (LDRT, 2 Gy × 10) or high-dose hypofractionated treatment (8 Gy × 2) in syngeneic mice. High-dose hRT did not affect peripheral and tumor-infiltrating CD8+ T-cells, reduced neoplastic accumulation of granulocyte-like myeloid suppressor cells, while T-reg lymphocytes were largely unaltered. Expression of IFN-responsive MHC class I peptides and PD-L1 were enhanced in tumors treated with 8 Gy × 2 compared to 2 Gy × 10 schedule. Functionally, tumor-specific CD8+-lymphocytes responses within tumor draining lymph nodes were enhanced following 8 Gy × 2 schedule but suppressed following 2 Gy × 10 irradiation [53]. When combined with PD-1 mAb, reversal of adaptive immune resistance was observed following 8 Gy × 2, but not following 2 Gy × 10, with subsequent enhancement of CD8+ cell-dependent primary and abscopal tumor response. These data strongly support that high-dose hRT preserves or enhances anti-tumor immunity compared to daily low-dose fractionated irradiation and, when combined with PD-1 inhibitor, reverses adaptive immune resistance, promotes anti-tumor immunity, and controls primary and distant lesions [53].
In order to determine the optimal dose for tumor and immunological response, Schaue et al. conducted a single fraction dose escalation study with doses ranging from 5 to 15 Gy and demonstrated that fractions of 7.5 Gy and above are immune-stimulatory, determining an increased number of tumor-reactive T-cells [54]. Instead a dose higher than 15 Gy in single fraction increased splenic T-reg fraction, while the same total dose fractionated boosted the number of effector T-cells in the spleen and decreased T-regs, with an optimal dose fractionation of 7.5 Gy × 2 [54]. Dewan et al. investigated three different regimens of radiotherapy (20 Gy × 1, 8 Gy × 3, or 6 Gy × 5) to obtain abscopal response in syngeneic mice models, with or without antiCTLA-4, finding that a significant AbE was only induced when RT was administered in fractionated schedules [55]. Additionally, observations by Vanpouille-Box, et al. suggest that AbE could only be achieved with hRT (8 Gy × 3) when combined with immune checkpoint inhibitors [32][33].
The systemic antitumor response of focal hRT combined with ICI may also be mitigated by the immunosuppressive properties of the non-irradiated distant tumors, that hinder T-cell entry and T-cell functioning [56][57]. In this regard, strategies that attempt to modulate the stroma of metastasis to improve T-cell infiltration are basic to invigorate the systemic antitumor response of combined hRT and immune checkpoint inhibitors. In fact, despite its very poor tumor-killing effect, LDRT is effective to T-cell recruitment [58][59]. Klug et al. have shown that LDRT (with one fraction of 2 Gy) may reprogram macrophages in TME, leading to the production of inducible nitric oxide synthase (iNOS), which normalizes tumor vasculature, thus promoting T-cell infiltration and enhancing the efficacy of adoptive T-cell therapy [58]. Moreover Yin et al. preclinically demonstrate that LDRT on established metastases, in combination with ICIs, significantly enhances the abscopal response to hRT treatment on the primary tumor compared to hRT/anti-PD1, hRT/LDRT, or LDRT/anti-PD1 combined treatments [60]. The enhanced abscopal effect was linked to an increased infiltration of CD8+ effector T-cells and an upregulated expression of T-cell-attracting chemokines, so localized LDRT to a second lesion should be indispensable for an enhanced systemic antitumor response, triggered by combined focal hRT and anti-PD1 therapy [60].
This preclinical model has been later confirmed by a post-hoc analysis of a 3 immunoradiation trial by Menon et al. in which patients, that received LDRT (1–20 Gy), either as scatter from high-dose radiation or from intentional treatment of a second isocenter with low-dose radiation, in association with high-dose radiation and immunotherapy, were evaluated for response [61]. The LDRT lesions were compared to those that received no radiation (<1 Gy total). They assessed that LDRT may increase systemic response rates of metastatic disease treated with high-dose radiation and ICI combinations, offering a clinical proof-of-principle of the ability of LDRT to polarize tumor macrophage in M1 subtype and shift TME from “cold” to “hot” [61][62].
This entry is adapted from the peer-reviewed paper 10.3390/immuno1040029
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. Cancer J Clin. 2020, 70, 7–30.
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postop-erative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952.
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; A Spencer, S.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28.
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127.
- Siddiqui, F.; Patel, M.; Khan, M.; McLean, S.; Dragovic, J.; Jin, J.-Y.; Movsas, B.; Ryu, S. Stereotactic Body Radiation Therapy for Primary, Recurrent, and Metastatic Tumors in the Head-and-Neck Region. Int. J. Radiat. Oncol. 2009, 74, 1047–1053.
- Orth, M.; Lauber, K.; Niyazi, M.; Friedl, A.; Li, M.; Maihöfer, C.; Schüttrumpf, L.; Ernst, A.; Niemöller, O.M.; Belka, C. Current concepts in clinical radiation oncology. Radiat. Environ. Biophys. 2014, 53, 1–29.
- Mole, R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241.
- Ashrafizadeh, M.; Farhood, B.; Musa, A.E.; Taeb, S.; Rezaeyan, A.; Najafi, M. Abscopal effect in radioimmunotherapy. Int. Immunopharmacol. 2020, 85, 106663.
- Marciscano, A.E.; Haimovitz-Friedman, A.; Lee, P.; Tran, P.T.; Tomé, W.A.; Guha, C.; Kong, F.-M.; Sahgal, A.; El Naqa, I.; Rimner, A.; et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 35–52.
- Ward, J. DNA Damage Produced by Ionizing Radiation in Mammalian Cells: Identities, Mechanisms of Formation, and Reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125.
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425.
- De Leve, S.; Wirsdorfer, F.; Jendrossek, V. Targeting the immunomodulatory CD73/adenosine system to improve the therapeutic gain of radiotherapy. Front. Immunol. 2019, 10, 698.
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015, 75, 2232–2242.
- Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Pilones, K.A.; García-Martínez, E.; Rudqvist, N.P.; Formenti, S.C.; Demaria, S. Barriers to radiation-induced in situ tumor vaccination. Front. Immunol. 2017, 8, 229.
- Darragh, L.B.; Oweida, A.J.; Karam, S.D. Overcoming resistance to combination ra-diation-immunotherapy: A focus on contrib-uting pathways within the tumor microenvironment. Front. Immunol. 2018, 9, 3154.
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 1992, 13, 265–270.
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555.
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Freeman Programmed Death-1 Ligand 1 Inter-acts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122.
- Matsuoka, Y.; Nakayama, H.; Yoshida, R.; Hirosue, A.; Nagata, M.; Tanaka, T.; Kawahara, K.; Sakata, J.; Arita, H.; Nakashima, H.; et al. Il-6 controls resistance to radiation by suppressing oxidative stress via thenrf2-antioxidant pathway in oral squamous cell carcinoma. Br. J. Cancer 2016, 115, 1234–1244.
- Ding, Q.; Lu, P.; Xia, Y.; Ding, S.; Fan, Y.; Li, X.; Han, P.; Liu, J.; Tian, D.; Liu, M. CXCL9: Evidence and contradictions for its role in tumor progression. Cancer Med. 2016, 5, 3246–3259.
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural killer cell accumula-tion in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008, 68, 8437–8445.
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015, 6, 7458.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111.
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology 2014, 3, e955691.
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745.
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548.
- Wang, J.; Zheng, H.; Sung, C.C.; Richter, K.K.; Hauer-Jensen, M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am. J. Pathol. 1998, 153, 1531–1540.
- Vigneron, N. Human tumor antigens and cancer immunotherapy. Biomed. Res. Int. 2015, 2015, 948501.
- Bhalla, N.; Brooker, R.; Brada, M. Combining immunotherapy and radiotherapy in lung cancer. J. Thorac. Dis. 2018, 10, S1447–S1460.
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642.
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon–Dependent Innate and Adaptive Immunity. Cancer Res. 2011, 71, 2488–2496.
- Vanpouille-Box, C.; Formenti, S.C.; DeMaria, S. TREX1 dictates the immune fate of irradiated cancer cells. OncoImmunology 2017, 6, e1339857.
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; DeMaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618.
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune me-diated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870.
- Deng, L.; Liang, H.; Burnette, B.; Weicheslbaum, R.R.; Fu, Y.X. Radiation and an- ti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology 2014, 3, e28499.
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800.
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11 2 Pt 1, 728–734.
- Verbrugge, I.; Hagekyriakou, J.; Sharp, L.L.; Galli, M.; West, A.; McLaughlin, N.M.; Duret, H.; Yagita, H.; Johnstone, R.W.; Smyth, M.J.; et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012, 72, 3163–3174.
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 2015, 3, 345–355.
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751.
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104.
- Latchman, Y.E.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; A Brown, J.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268.
- Callahan, M.K.; Wolchok, J.D. At the Bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 41–53.
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377.
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radio-therapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468.
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nat. Cell Biol. 2005, 439, 682–687.
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically En-hances Antitumor Effect in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1085–1097.
- Hettich, M.; Lahoti, J.; Prasad, S.; Niedermann, G. Checkpoint Antibodies but not T Cell–Recruiting Diabodies Effectively Synergize with TIL-Inducing γ-Irradiation. Cancer Res. 2016, 76, 4673–4683.
- Frey, B.; Rückert, M.; Weber, J.; Mayr, X.; Derer, A.; Lotter, M.; Bert, C.; Rödel, F.; Fietkau, R.; Gaipl, U.S. Hypofractionated Irradiation Has Immune Stimulatory Potential and Induces a Timely Restricted Infiltration of Immune Cells in Colon Cancer Tumors. Front. Immunol. 2017, 8, 231.
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990, 123, 224–227.
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta. 2014, 1846, 121–129.
- Zhang, X.; Niedermann, G. Abscopal Effects with Hypofractionated Schedules Extending Into the Effector Phase of the Tumor-Specific T-Cell Response. Int. J. Radiat. Oncol. 2018, 101, 63–73.
- Morisada, M.; Clavijo, P.E.; Moore, E.; Sun, L.; Chamberlin, M.; Van Waes, C.; Hodge, J.W.; Mitchell, J.B.; Friedman, J.; Allen, C.T. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. OncoImmunology 2017, 7, e1395996.
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing Tumor Immunity With Fractionated Radiation. Int. J. Radiat. Oncol. 2012, 83, 1306–1310.
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388.
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135.
- Demaria, S.; Golden, E.B.; Formenti, S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015, 1, 1325–1332.
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602.
- De Palma, M.; Coukos, G.; Hanahan, D. A new twist on radiation oncology: Low-dose irradiation elicits immunostimulatory macrophages that unlock barriers to tumor immunotherapy. Cancer Cell 2013, 24, 559–561.
- Yin, L.; Xue, J.; Li, R.; Zhou, L.; Deng, L.; Chen, L.; Zhang, Y.; Li, Y.; Zhang, X.; Xiu, W.; et al. Effect of Low-Dose Radiation Therapy on Abscopal Responses to Hypofractionated Radiation Therapy and Anti-PD1 in Mice and Patients With Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2020, 108, 212–224.
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.; Cortez, M.A.; Erasmus, J.J.; De Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237.
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, P.J.; et al. Local radiotherapy and granulo-cyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803.
