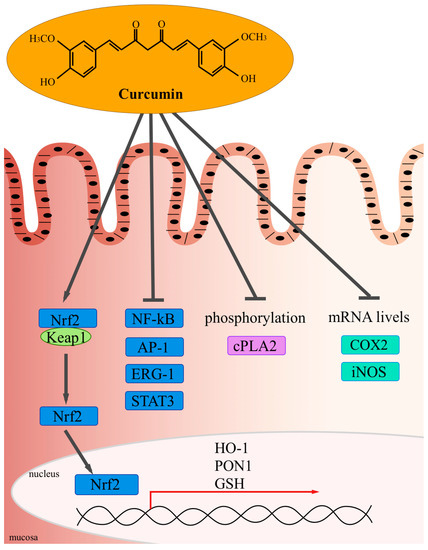
The conventional approach to IBD aims to induce and maintain clinical remission free of corticosteroids, thus minimizing the impact on quality of life.(1) Currently, corticosteroids, sulfasalazine, mesalamine (5-ASA), and immunomodulators are treatment options for patients with IBD. Studies indicate that a substantial proportion of patients do not fully respond to conventional treatment for IBD or that its efficacy wanes over time.(2) Corticosteroid resistance/refractoriness rates range from 8.9% to 25% in individuals with IBD.(3, 4)
Identifying safe and effective therapeutic agents for complementary therapy remains an unmet need for these patients. Curcumin also acts by inhibiting the activity of pro-inflammatory proteins such as activated protein-1, peroxisome proliferator-activated receptor gamma, signal translators, and transcription activators, as well as the expression of b-catenin, cyclooxygenase 2, 5-lipoxygenase, and inducible nitric oxide synthase isoform, which play a key role in inflammation.(5) In addition, it acts by blocking the binding between TNF-α and its receptor, preventing the perpetuation of inflammation caused by this cytokine.(6) Curcumin's excellent anti-inflammatory profile makes it a promising therapeutic agent in the treatment of IBD.
- Inflammatory Bowel Disease
- Curcumin
- Complementary Therapies
- Phytotherapy
1. Introduction
Inflammatory bowel disease (IBD) is a chronic condition that affects the relapsing gastrointestinal tract, with periods of exacerbation and remission. Its main forms of presentation are ulcerative colitis (UC) and Crohn's disease (CD). Its etiopathogenesis is believed to be due to loss of tolerance to the intestinal microbiota associated with marked immune response and environmental factors in genetically susceptible individuals.[1]
The conventional approach to IBD aims to induce and maintain clinical remission free of corticosteroids, thus minimizing the impact on quality of life. It is worth mentioning that conventional treatment causes numerous side effects due to the marked immune response suppression, which negatively impact the quality of life of these individuals.[2]
Identifying safe and effective therapeutic agents for complementary therapy remains an unmet need for these patients. Curcuma longa is a plant from the Zingiberaceae family that is native to India and Southeast Asia and is well known in Asian cultures. Known commonly as turmeric, it has long been used in Ayurvedic medicine to treat inflammatory diseases. It has attracted the attention of researchers because of its compounds, called curcuminoid pigments, which are polyphenols with important medicinal properties.[3]
The mechanism of its anti-inflammatory action deemed to be the most relevant is the inhibition of NF-κB by blocking IκB kinase, which prevents cytokine-mediated phosphorylation and the degradation of IκB, an NF-κB inhibitor, thereby inhibiting the expression of pro-inflammatory cytokines (IL-1, IL-6, and TNF).[4] Curcumin also acts by inhibiting the activity of pro-inflammatory proteins such as activated protein-1, peroxisome proliferator-activated receptor gamma, signal translators, and transcription activators, as well as the expression of b-catenin, cyclooxygenase 2, 5-lipoxygenase, and inducible nitric oxide synthase isoform, which play a key role in inflammation.[5] In addition, it acts by blocking the binding between TNF-α and its receptor, preventing the perpetuation of inflammation caused by this cytokine.[6]
Source: Vecchi Brumattiet al., 2014[5].
2. Discussion
Five randomized clinical trials [7][8][9][10][11] reported that curcumin was able to reduce the symptoms of the disease, achieve clinical remission, and/or prevent relapse when used as a complementary therapy to mesalamine. In Masoodi's study,[10] in addition to a significant reduction in SCCAI, a higher proportion of the intervention group patients reported improved general well-being and decreased fecal urgency than did the patients from the placebo group after four weeks. Curcumin was well tolerated in all the RCTs and was not associated with any serious side effects. Only Hanai et al.[7] reported some mild AE, such as abdominal distension, nausea and increased number of bowel movements in a Japanese population. These non-specific symptoms may be related to factors not controlled by the researchers, such as dietary factors (lactose, fodmaps, gluten) and the presence of associated functional diseases, such as IBS (irritable bowel syndrome). Therefore, it cannot be confirmed that these symptoms experienced by only seven of 89 patients are related to curcumin use.
The study by Kedia et al.,[12] using lower doses of oral capsule curcumin than other studies,[7][9][11] which used 2,000mg/day, 3,000mg/day and 1,500mg/day respectively, was the only one in which no significant difference was found between clinical remission and endoscopic remission rates in the intervention and placebo group (UCDAI). It is understood that very low doses of curcumin may not achieve the desired effect unless administered locally in the form of an enema, as in the study by Singla et al.,[8] or in more bioavailable nanoformulations such as SinaCurcumin®, used in the study by Masoodi et al.[10]
The Food and Drug Administration states that curcumin is “generally recognized as safe” and has no known toxic effects. According to the FAO/WHO Joint Food Additives Expert Committee and the European Food Safety Authority, the acceptable daily intake of curcumin is 0–3mg/kg/day.[13][14] Lao et al.[15] administered 500–12000 mg curcumin (95% standardized extract of curcumin) in healthy subjects to examine its maximum tolerance and safety dose, and found that ingestion of up to 12g/day curcumin brought about no ill effects. Based on this recommendation, a healthy 70 kg individual could consume 4–10g turmeric powder, or 2 tablespoons, per day, which is well above the usual consumption in western countries. The bioavailability of orally ingested unformulated curcumin is low. High doses of curcumin (2–4g) are usually required to improve bioavailability due to its hydrophobic nature, but recently, in addition to nanoformulations, studies have been conducted into a self-micro emulsifying drug delivery system (SMEDDS) for curcumin: hydrophilic drug droplets that can diffuse easily into the bloodstream, resulting in higher intraintestinal concentrations than when conventional curcumin is used.[16] Although SMEDDS has been used successfully in a study, current evidence on its usefulness is still scant.
In a single-blind crossover study in healthy adult, the bioavailability of the curcumin micellar formulation was found to be 185 times higher than that of the same dose of unformulated curcumin.[17] Despite promising results concerning curcumin micellization, the study was conducted on healthy subjects; there are no studies on individuals with IBD. This nanometer-sized drug delivery system could become an effective strategy for treating IBD.[18] Another strategy for improving bioavailability, the use of piperine (a component of black pepper, Piper nigrum, and long pepper, Piper longum) as an adjunct to curcumin, has been described for a long time. Shoba et al.[19] demonstrated that 20mg piperine administered concomitantly with 2g curcumin increases the bioavailability of curcumin 20-fold in humans. One experimental study by Li et al.(21) using CUR-PIP-SMEDDS (an emulsified curcumin and piperine formulation) reported that the administration of this formulation through enema in rats had an effect similar to 5-ASA in maintaining remission in DSS-induced colitis.
3. Conclusions
Efforts are currently being made by different research groups to improve curcumin bioavailability, and further efforts will be needed to answer questions related to curcumin therapy in IBD. New well-designed, long-term RCTs with a large enough sample size to demonstrate clinically significant effects and to determine the efficacy of new pharmaceutical formulations are required. The findings suggest that curcumin may be a safe, effective therapy for maintaining or inducing UC remission when administered with standard treatments; the same cannot be said for CD, due to the absence of RCTs with a low risk of bias investigating patients with this condition.
This entry is adapted from the peer-reviewed paper 10.3390/nu12082296
References
- Sozaburo Ihara; Yoshihiro Hirata; Kazuhiko Koike; TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. Journal of Gastroenterology 2017, 52, 777-787, 10.1007/s00535-017-1350-1.
- Marcus Harbord; Rami Eliakim; Dominik Bettenworth; Konstantinos Karmiris; Konstantinos Katsanos; Uri Kopylov; Torsten Kucharzik; Tamas Molnar; Tim Raine; Shaji Sebastian; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. Journal of Crohn's and Colitis 2017, 11, 769-784, 10.1093/ecco-jcc/jjx009.
- Betül Kocaadam; Nevin Sanlier; Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical Reviews in Food Science and Nutrition 2015, 57, 2889-2895, 10.1080/10408398.2015.1077195.
- Manjeshwar Shrinath Baliga; Nandhini Joseph; Marikunte V. Venkataranganna; Arpit Saxena; Venkatesh Ponemone; Raja Fayad; Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food & Function 2011, 3, 1109-1117, 10.1039/c2fo30097d.
- Liza Vecchi Brumatti; Annalisa Marcuzzi; Paola Maura Tricarico; Valentina Zanin; Martina Girardelli; Anna Monica Bianco; Curcumin and Inflammatory Bowel Disease: Potential and Limits of Innovative Treatments. Molecules 2014, 19, 21127-21153, 10.3390/molecules191221127.
- Hazem Khalaf; Jana Jass; Per-Erik Olsson; Differential cytokine regulation by NF-κB and AP-1 in Jurkat T-cells. BMC Immunology 2010, 11, 26-26, 10.1186/1471-2172-11-26.
- Hiroyuki Hanai; Takayuki Iida; Ken Takeuchi; Fumitoshi Watanabe; Yasuhiko Maruyama; Akira Andoh; Tomoyuki Tsujikawa; Yosihihide Fujiyama; Keiichi Mitsuyama; Michio Sata; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clinical Gastroenterology and Hepatology 2006, 4, 1502-1506, 10.1016/j.cgh.2006.08.008.
- Vikas Singla; Venigalla Pratap Mouli; Sushil K Garg; Tarun Rai; Bikash Narayan Choudhury; Prashant Verma; Rachana Deb; Veena Tiwari; Sarika Rohatgi; Rajan Dhingra; et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study.. Journal of Crohn’s and Colitis 2014, 8, 208-214, 10.1016/j.crohns.2013.08.006.
- A. Lang; Nir Salomon; Justin C.Y. Wu; Uri Kopylov; Adi Lahat; Ofir Har-Noy; Jessica Y.L. Ching; Pui Kuan Cheong; Benjamin Avidan; Dorit Gamus; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clinical Gastroenterology and Hepatology 2015, 13, 1444-1449.e1, 10.1016/j.cgh.2015.02.019.
- Mohsen Masoodi; Mohammad Ali Mahdiabadi; Marjan Mokhtare; Shahram Agah; Amir Hossein Faghihi Kashani; Amir Mansoor Rezadoost; Mohammad Sabzikarian; Atefeh Talebi; Amirhossein Sahebkar; The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: A randomized double-blind controlled trial. Journal of Cellular Biochemistry 2018, 119, 9552-9559, 10.1002/jcb.27273.
- Narges Sadeghi; Anahita Mansoori; Aliakbar Shayesteh; Seyed Jalal Hashemi; The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytotherapy Research 2019, 34, 1123-1133, 10.1002/ptr.6581.
- Saurabh Kedia; Vikram Bhatia; Sandeep Thareja; Sushil Garg; Venigalla Pratap Mouli; Sawan Bopanna; Veena Tiwari; Govind Makharia; Vineet Ahuja; Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World Journal of Gastrointestinal Pharmacology and Therapeutics 2016, 8, 147-154, 10.4292/wjgpt.v8.i2.147.
- European Food Safety Authority; Refined exposure assessment for curcumin (E 100). EFSA Journal 2014, 12, 3876, 10.2903/j.efsa.2014.3876.
- CURCUMIN Chemical and Technical Assessment (CTA) . http://www.fao.org. Retrieved 2020-8-17
- Christopher D. Lao; Iv Mack T. Ruffin; Daniel P. Normolle; Dennis D Heath; Sandra I Murray; Joanne M Bailey; Martha E Boggs; James Crowell; Cheryl L. Rock; Dean E Brenner; et al. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine 2006, 6, 10-10, 10.1186/1472-6882-6-10.
- Rupa Banerjee; Amulya Penmetsa; Kavitha Medaboina; Ganesh Girish Boramma; Sarika Amsrala; Duvur N. Reddy; Novel Bio-Enhanced Curcumin with Mesalamine for Induction of Remission in Mild to Moderate Ulcerative Colitis. Gastroenterology 2017, 152, S587, 10.1016/s0016-5085(17)32111-x.
- Christina Schiborr; Alexa Kocher; Dariush Behnam; Josef Jandasek; Simone Toelstede; Jan Frank; The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Molecular Nutrition & Food Research 2014, 58, 516-527, 10.1002/mnfr.201300724.
- Masashi Ohno; Atsushi Nishida; Yoshihiko Sugitani; Kyohei Nishino; Osamu Inatomi; Mitsushige Sugimoto; Masahiro Kawahara; Akira Andoh; Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999, 10.1371/journal.pone.0185999.
- Guido Shoba; David Joy; Thangam Joseph; M. Majeed; R. Rajendran; P. Srinivas; Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Medica 1998, 64, 353-356, 10.1055/s-2006-957450.