Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
Lithium-ion battery is a rechargeable battery, which mainly relies on the movement of lithium ions between the positive and negative electrodes to work. Lithium-ion batteries use an intercalated lithium compound as an electrode material. Currently, the main common cathode materials used as lithium ion batteries are: lithium cobalt oxide, lithium manganate, lithium nickelate and lithium iron phosphate.
- Li-ion batteries
1. The Components of Li-Ion Batteries
Due to their high power and energy density, long life span, very affordable work temperatures, high voltage, low volatility rates, and other positive properties, lithium-ion batteries are used in a wide range of applications. The gradual improvement in their performance and a significant reduction in the cost of these types of batteries has led to their increasing exploitation in moveable electronic devices, such as mobile phones, tablets, and EVs. However, the high speed of technology growth in the various domains has led to the need for cheaper, more reliable, and better-performing batteries [1]. Figure 2 shows the components of a family of Li-ion batteries and typical Li-ion battery packs. Moreover, Lithium-Sulfur (Li-S), due to its advantages, will be a new family of Li-ion batteries [2][3]. According to Figure 2, there are five main components in the Li-ion batteries: anode, cathode, electrolyte, separator, and current collector.
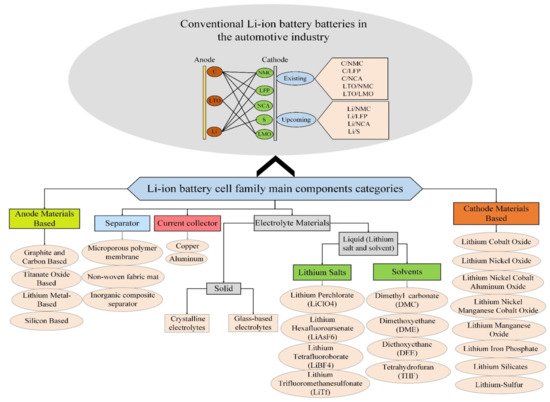
Figure 2. Components of the conventional Li-ion battery cells in the automotive industry.
Different types of rechargeable batteries have also been introduced, and several of them have been given more attention for proper performance in EVs. Different types of technologies are frequently used for battery cells on Carbon (C) Anode material Lithium-Iron-Phosphate (LFP), Nickel-Manganese-Cobalt (NMC), Nickel-Cobalt-Aluminum-Oxide (NCA), Lithium-ion Manganese Oxide (LMO), and Lithium Titanium Oxide (LTO) from the cathode side [4].
2. The Physical Implementation of Li-Ion Batteries
There are three main shapes for the individual cell used in the EV’s battery pack [5]: cylindrical, prismatic, and pouch. Table 1 compares the Li-ion batteries from the physical perspective. According to Table 1, the main factors for comparing the different Li-ion batteries from a shape perspective are energy density, heat management, mechanical strength, electrode arrangement, and specific energy. In general, the prismatic cell has become well known because of its better performance.
Table 1. Li-ion battery comparison from the physical implementation perspective.
| Name | Cylindrical | Prismatic | Pouch |
|---|---|---|---|
| Shape |  |
 |
 |
| Arrangement of electrode | Wound | Wound | Wound |
| Mechanical stability | Best | Good | Bad |
| Heat management | Bad | Good | Good |
| Specific energy | Good | Good | Best |
| Energy density | Good | Best | Good |
3. Definitions Regarding Li-Ion Batteries
3.1. Voltage and Capacity
The two most important parameters in a Li-ion battery are voltage and capacity. The voltage, expressed in volts, measures the electrochemical potential available in the cell, which is determined by the type of active material contained in the cell. Moreover, the mass of the active material determines the cell capacity. The capacity is a measure (in Ah) of the charge which can be stored in the battery and indicates the energy that can be extracted from the battery. The nominal rated capacity is usually defined by the battery manufacturers and determined under specific conditions. However, different operating conditions and battery aging can strongly affect the real capacity of the battery, which reduces the available stored energy [6].
3.2. State of Charge and Depth of Discharge
The state of charge (SoC) of a battery can be described as the proportion between the charge available at a specific time and the total available charge when the battery is fully charged, and is expressed in percentage and varies between 0 and 100. In Li-ion barriers, there are three main areas of operations from the SoC perspective: exponential area, nominal area, and discharge area.
If we look at how battery capacity usually performs server timing, we can see three distinct phases. In the first stage, the rate of capacity fade rapidly decays as the solid electrolyte interferes with stabilizers and becomes less reactive to the electrolyte. Although battery degradation is a non-linear process, the second stage (until the knee point) represents the linear region. Once we enter the knee, the degradation mechanisms occur and result in an increasing rate of capacity loss and cell failure occurs very quickly.
3.3. C-Rate
The unit of electric current is the ampere (A), but an alternative and potentially more intuitive measure of a battery with respect to its current comes from the definition of the C-rate [7]. The C-rate determines the current required to completely charge and discharge a battery in a determined period. For instance, a battery with a nominal capacity of 70 Ah can be entirely charged in one hour applying a current of 70 A (C-rate = 1C), in two hours at 35 A (C-rate = C/2), or in half an hour at 140 A (C-rate = 2C) [8].
3.4. Internal Resistance
Li-ion batteries’ internal resistance is conditional on many factors; therefore, it cannot be considered as a constant. It is generally used to model the voltage drop of the cell under load conditions and the associated power dissipation. There are many different definitions of battery internal resistance present in the literature. The common property in these definitions is the internal resistance, which acts in opposition to the current flow within the battery. The internal resistance is defined as diffusion polarization resistance, and an activation which is the most significant voltage drop in the battery [9].
3.5. Energy and Power
The energy of a battery is defined as the capacity multiplied by its voltage. The nominal energy depends on the intrinsic electrochemical characteristics of the cell. It is essential to understand that the energy storage capabilities of a battery can vary significantly from their nominal values due to various factors, such as aging, temperature, and operating conditions [10].
The energy, power, cost, safety, and lifetime are the most important parameters to define the performance of a battery. One standard method to compare the performance of batteries, and more generally energy storage devices, is the Ragone plot [11]. Christen and Carlen characterized different Ragone curves for different types of energy storage devices (ESD), highlighting the difference between inductive ESDs (SMEs or flywheels), where energy increases with power, and capacitive ESDs (capacitors and batteries), where energy decreases with power [12][13]. While batteries are the ESD with the highest available energy density (especially Li-ion batteries), they are not yet able to completely fulfill the US Advanced Battery Consortium (USABC) requirements for EV applications [11][14].
This entry is adapted from the peer-reviewed paper 10.3390/su132111688
References
- Das, U.K.; Shrivastava, P.; Tey, K.S.; Bin Idris, M.Y.I.; Mekhilef, S.; Jamei, E.; Seyedmahmoudian, M.; Stojcevski, A. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 134, 110227.
- Chung, S.H.; Tancogne-Dejean, T.; Zhu, J.; Luo, H.; Wierzbicki, T. Failure in lithium-ion batteries under transverse indentation loading. J. Power Sources 2018, 389, 148–159.
- Gandoman, F.H.; Ahmadi, A.; Van den Bossche, P.; Van Mierlo, J.; Omar, N.; Nezhad, A.E.; Mavalizadeh, H.; Mayet, C. Status and future perspectives of reliability assessment for electric vehicles. Reliab. Eng. Syst. Saf. 2019, 183, 1–16.
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549.
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074.
- Piernas Muñoz, M.J.; Castillo Martínez, E. Introduction to batteries. In Prussian Blue Based Batteries; Springer: New York, NY, USA, 2018; pp. 1–8.
- State of Charge (SOC) Determination. Available online: https://www.mpoweruk.com/soc.htm (accessed on 21 October 2021).
- What Is C-Rate? Available online: https://batteryuniversity.com/learn/article/what_is_the_c_rate (accessed on 21 October 2021).
- Wang, D.; Bao, Y.; Shi, J. Online lithium-ion battery internal resistance measurement application in state-of-charge estimation using the extended kalman filter. Energies 2017, 10, 1284.
- Fang, Q.; Wei, X.; Dai, H. A remaining discharge energy prediction method for lithium-ion battery pack considering SOC and parameter inconsistency. Energies 2019, 12, 987.
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939.
- Mostafa, M.H.; Aleem, S.H.E.A.; Ali, S.G.; Abdelaziz, A.Y.; Ribeiro, P.F.; Ali, Z.M. Robust energy management and economic analysis of microgrids considering different battery characteristics. IEEE Access 2020, 8, 54751–54775.
- Mostafa, M.H.; Abdel Aleem, S.H.E.; Ali, S.G.; Ali, Z.M.; Abdelaziz, A.Y. Techno-economic assessment of energy storage systems using annualized life cycle cost of storage (LCCOS) and levelized cost of energy (LCOE) metrics. J. Energy Storage 2020, 29, 101345.
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128.
This entry is offline, you can click here to edit this entry!
