O-GlcNAcylation is an emerging potential mechanism for cancer cells to induce proliferation and progression of tumor cells and resistance to chemotherapy.
1. Introduction
O-GlcNAcylation affects many diseases, including diabetes, diabetic nephropathy, and neurodegenerative disease such as Alzheimer disease [11,12,13,14,15]. In recent years, several studies have addressed the role of protein O-GlcNAcylation in various types of cancer, including the impact of O-GlcNAcylation in proliferation, angiogenesis, and metastasis of cancer cells [16,17].
2. O-GlcNAcylation and Cancer
2.1. O-GlcNAcylation and Metastasis
Several cancers, notably breast, colon, pancreas, liver, and lung cancers, have been associated with elevated O-GlcNAcylation [
54,
55]. The Cancer Genome Atlas datasets show the aberrant levels of OGT in both adenocarcinoma and squamous cell carcinoma of lung [
56]. This results in invasion, metastasis, and angiogenesis of lung cancer cells by activating transcription factors such as Notch receptor 1 (Notch1) and nuclear factor erythroid 2-related factor (Nrf2) [
57,
58]. Notch-dependent metastasis is potentially modulated via O-GlcNAcylation [
57,
59].
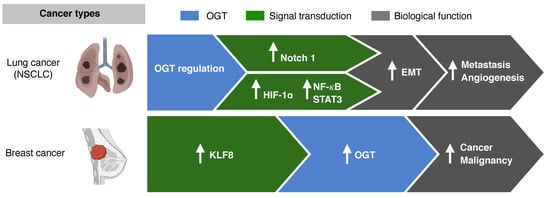
In NSCLC, O-GlcNAcylation mediates and sustains the epithelial mesenchymal transition (EMT) [
60]. EMT markers, such as E-cadherin and vimentin, are suppressed when the O-GlcNAcylation levels are high (
Figure 3) [
61,
62]. Cancer cells that undergo EMT have more aggressive and invasive features due to their ability to migrate [
63]. Other transcriptional factors, such as hypoxia-inducible factor(HIF) -1α, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and signal transducer and activator of transcription 3 (STAT3), are also activated via O-GlcNAcylation, resulting in cancer invasion and metastasis in cancers such as NSCLC, cervical cancer, and head and neck cancer [
59,
64,
65].
Figure 3. O-GlcNAcylation and metastasis. In non-small cell lung cancer (NSCLC), the epithelial mesenchymal transition (EMT) and transcriptional factors such as hypoxia-inducible factor-1α (HIF) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) are mediated via O-GlcNAcylation. In breast cancer, the activation of Krüppel-like factor 8 (KLF8) results in OGT activation.
In vitro and in vivo studies on breast cancer cell lines have shown that O-GlcNAc transferase regulates cancer stem-like potential [54]. Krüppel-like factor 8 (KLF8) acts as a novel regulator in mammosphere formation, and activation of KLF8 resulted in activation of OGT in xenograft tumors in vivo. Breast cancers with KLF8 showed worse OS than breast cancers without KLF8 expression. The role of OGT in the regulation of cancer-stemness and tumor metastasis, as seen in breast cancer, may potentially be targeted to overcome resistance to chemotherapy.
2.2. O-GlcNAcylation and Receptor Tyrosine Kinase
Activation of downstream pathways of RTK, such as KRAS and epidermal growth factor receptor (EGFR), is associated with an increased glucose flux via the HBP pathway [
66,
67]. Approximately 90% of the pancreatic ductal adenocarcinoma (PDAC) is associated with KRAS [
68]. Increased cellular O-GlcNAcylation initiates reduction in ribonucleotide reductase activity and dNTP pools, resulting in genomic alterations, including
KRAS mutations in PDAC [
66]. Similarly, O-GlcNAcylation is associated with tumorigenesis in
KRAS-mutant lung cancer [
67]. In mouse models, the upregulation and downregulation of O-GlcNAcylation significantly accelerated and delayed
KrasG12D lung tumorigenesis, respectively.
EGFR mutations are also crucial in NSCLC, because more than 60% of patients diagnosed with NSCLC harbor
EGFR mutations, and treatment with tyrosine kinase inhibitors (TKIs) is preferred over chemotherapy or immunotherapy for these subset of patients [
69,
70]. Contrary to the cell lines of cervical adenocarcinoma, the cell lines of lung adenocarcinoma are associated with EGFR O-GlcNAcylation in the serine and/or threonine residue(s) [
71]. The PTM of EGFR via O-GlcNAcylation may possibly be tumor-specific, and warrants further exploration.
2.3. O-GlcNAcylation and Resistance to Chemotherapy
In lung carcinoma cells, hyper-O-GlcNAcylation is associated with cisplatin resistance via the regulation of either p53 or c-Myc [
72]. O-GlcNAcylation of p53 and cMyc results in p53 instability due to ubiquitin-mediated proteasomal degradation and inhibition of c-Myc ubiquitination and degradation, respectively. Recent in vitro and in vivo studies also showed that protein O-GlcNac modification of p53 or c-Myc affects the anti-tumor activity of cisplatin in NSCLC cell lines [
73]. Treatment with cisplatin increased the activities of OGT and OGA and decreased the activity of AMP-activated protein kinase. However, inhibition of OGT and OGA by altering O-GlcNAc levels did not result in an increased sensitivity of cisplatin in lung cancer cells.
In many types of cancers, cisplatin is the key chemotherapeutic in both the adjuvant and palliative settings [
74]. Identifying the potential mechanism behind cisplatin resistance is an unmet need for patients with cancers, as most of the patients experience disease progression with chemotherapeutic agents [
75]. In other cancer cell lines, such as hepatocellular carcinoma, inhibition of O-GlcNAc transferase resulted in enhancement of apoptosis by doxorubicin [
76]. Further studies are warranted to elucidate the role of O-GlcNAcylation in the resistance to chemotherapeutic agents used in cancers such as navelbine, pemetrexed, carboplatin, and docetaxel, as well as potential therapeutic agents targeting hyper-O-GlcNAcylation.
2.4. O-GlcNAcylation as Prognostic Marker
Several studies have shown that cancers, including prostate and colorectal cancers, harboring hyper-O-GlcNAcylation are associated with worse prognosis [
77,
78]. In squamous cell lung cancer, O-GlcNAcylation and increased OGT levels were observed in lung cancer cells compared with the adjacent lung tissue [
78]. The expression of OGT in patients with stages II, III, and IV lung adenocarcinomas was higher than that in patients with stage I lung adenocarcinoma [
79]. In this study, stage I patients with high OGT expression had shorter recurrence-free survival (RFS) and poor OS. Multivariate analysis revealed that high OGT was a prognostic factor for both RFS and OS, indicating OGT as a potential biomarker in early-stage lung adenocarcinoma. The clinical significance of O-GlcNAcylation is yet to be determined with larger prospective cohorts and validation studies.
3. O-GlcNAcylation and Immune Responses in Cancer
3.1. Overview of Immune System and O-GlcNAcylation
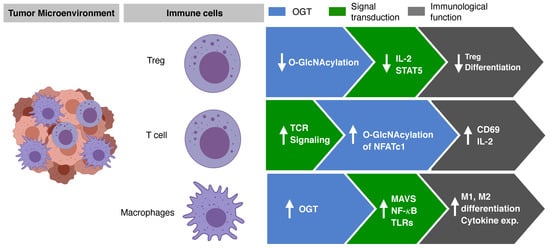
O-GlcNAcylation is highly related to the immune surveillance in the tumor microenvironment (
Figure 4). The metabolic shift in immune cells affects T-cell activation and differentiation. Increased amounts of energy metabolites, such as glucose and amino acids, are required for T cells. Specifically, glutamine uptake is essential in activated T cells. Initially, glutamine is converted to a source of oxaloacetate in the tricarboxylic acid cycle (TCA) cycle. The acetyl-CoA is generated by the metabolism of TCA cycle, which allows for greater fatty acid synthesis. The produce of this metabolic pathway serves as a substrate in the HBP [
80]. The role and function of O-GlcNAcylation for the two important immune cells constituting the tumor microenvironment have been studied. The effects of O-GlcNAcylation in the tumor microenvironment include dealing with the differentiation and signaling mechanisms of T cells and differentiation and activation of macrophages.
Figure 4. O-GlcNAcylation regulates immune cells activation and differentiation. O-GlcNAcylation is associated with the immune surveillance in tumor microenvironment.
3.2. T Cell Activation and Differentiation Regulated by O-GlcNAcylation
In the thymus, lymphocyte development and activation were observed with increased O-GlcNAc level [
81]. This finding suggested that the modification of O-GlcNAc occurred during the early stages of T lymphocyte activation. In addition, the OGT knockdown experiment showed an impairment of interleukin (IL)-2 production in the T cells. Furthermore, ZAP70, SHIP1, and LCK were identified as substrates of O-GlcNAc that regulate TCR signaling [
82].
In general, anticancer immune responses are regulated by cellular immunity, and in particular, Th1-type cells activate surrounding cytotoxic T lymphocytes, helping the cytokines kill the tumor cells more effectively. In addition, Th2 and Th17 cells have different roles. Th2 cells induce the activation of B cells through humoral immunity but can reduce the Th1 immune response. Th17 cells promote the differentiation of fibroblast-like cells. Treg cells play an important role in reducing the immune response by depletion of IL-2, the expression of transforming growth factor beta and IL-10, and CTLA-4. Interestingly, it has been reported that O-GlcNAc is involved in the differentiation of Th cells. When TMG, a drug capable of inhibiting OGA, was administered to experimental animals, the levels of O-GlcNAc decreased [
83]. Whether Th17 is a friend or foe in terms of tumor immune response is still unclear. In another study, O-GlcNAcylation was found to regulate Foxp3 [
84]. Treg is well known as an important regulator of antitumor effect in tumor immunity. In Treg cells deficient in O-GlcNAc, it was confirmed that Foxp3 expression was reduced; and therefore, Treg functions were not effective, thus explaining the regulation of IL-2/STAT5 by O-GlcNAc. A recent study reported that STAT3 and the signal transducer and activator of transcription 5 (STAT5), which play important roles in Th17 cell differentiation and Treg differentiation, respectively, are important factors influencing T cell differentiation and can be regulated by O-GlcNAc [
84,
85]. In addition, O-GlcNAcylation of key signaling proteins that play an important role in T cells, such as nuclear factor of activated T cells (NFAT), affects the activation and function of T cells [
86]. TCR in T cells acts as an important sensor that can detect and kill major histocompatibility complex molecules when presented with a malformed molecule such as cancer. TCR activation rapidly induced O-GlcNAcylation of NFATc1, and O-GlcNAcylated protein was observed in the nucleus within 5 min [
86]. These results show that O-GlcNAcylation plays an important role in gene regulation of TF protein. This was verified through the reduction in TCR-induced production of IL-2 and activation markers such as CD69 through the inhibition of OGT [
87].
3.3. Macrophage Differentiation and Activation by O-GlcNAcylation
Several articles have reported that a normal level of OGT increases HBP, and that O-GlcNAcylation increases mitochondrial antiviral-signaling proteins (MAVS) to enhance innate immune response. O-GlcNAcylation plays an important role in attenuating infection with vesicular stomach virus [
91]. O-GlcNAcylation enhances M1 macrophage polarization and inflammatory immune response [
85]. In contrast, it was reported that the activity of HBP plays an important role in M2 macrophage differentiation. The N-glycosylation pathway plays an important role in activating CD206 and CD301, which are important markers of M2 macrophages in metabolic function [
92]. Several studies have demonstrated that O-GlcNAcylation is involved in the activation of M1 and M2 macrophages. OGT increases the activity of lipopolysaccharide (LPS)-stimulated NF-κB and the expression of iNOS gene through mSin3a [
93]. In addition, it has been confirmed in microglia cells, that c-Rel and p65 are regulated by O-GlcNAcylation. However, it has also been reported that strong O-GlcNAcylation affects the differentiation and activity of macrophages by inhibiting NF-κB p65 signaling [
90]. This response is closely related to the response to the Toll-like receptor (TLR), which is an important function of macrophages. TLR4 signaling showed decreased activity by O-GlcNAcylation, revealing that O-GlcNAcylation is an important mechanism to regulate the innate immune response. LPS signaling increase due to lack of OGT is closely related to O-GlcNAcylation of RIPK3, which affects the phosphorylation of NF-κB and ERK. Phosphorylation of RIPK3 is enhanced in the absence of OGT, resulting in increased NF-κB and ERK signals. In addition, the activation of RIPK1, which affects necroptosis, is also associated with O-GlcNAcylation [
94]. Activation of NOD2, which plays an important role in the innate immune response, promotes the expression and secretion of cytokines and chemokines. NOD2 is post-translationally modified by O-GlcNAcylation, and its stability and activity is mediated by O-GlcNAcylation [
95].
3.4. O-GlcNAcylation and Tumor Microenvironment
Tumor microenvironment (TME) consists of various cells such as cancer cells, vascular endothelial cells, T cells, NK cells, macrophages, fibroblasts, and dendritic cells [
96,
97]. TME is a fairly complex system, including the metabolic interactions of various cells, and the direct interaction between cancer cells and immune cells. Macrophages and T cells are crucial in the TME [
98,
99]. Macrophages are involved in the role of tumor-antigen presentation, and immunosuppression in TME. Macrophages differentiate into M1- and M2-type macrophages, and occupy a high proportion in TME [
97]. M1 macrophages are activated by stimuli such as external pathogens and interferon gamma, and have inflammation effects by secreting IL-12, thereby creating a tumor suppressive environment [
100]. On the contrary, M2 macrophages are activated by IL-4, IL-10, and IL-13, and are known to have anti-inflammatory and immune suppressive effects on immune cells.
To overcome the immune suppressive TME created by M2 macrophages, various therapeutic agents are being developed to inhibit M2-type macrophage differentiation or increase the ratio of M1-type macrophages [
101,
102]. OGT may be a promising potential therapeutic target since OGT in macrophages is important in the regulation of phosphorylation of NF-kB, and the expression of iNOS and pro-inflammatory cytokine genes [
93]. Further elucidation on the role of O-GlcNAcylation in M1 and M2 will help pave the way for OGT as a therapeutic target.
T cells also play a critical role in tumor suppression, and kill tumors by directly reacting with tumor antigens expressed on the surface of cancer cells (epitope-MHC complex) [
103]. O-GlcNAcylation may function in the regulation of T-cell receptor (TCR) and T lymphocyte differentiation [
104]. On the other hand, T cells inhibit the differentiation of immune cells, thereby reducing anti-tumor immune responses by OGT-induced T reg [
84]. The dual mechanism of O-GlcNAcylation in T cells requires comprehensive immunological pre-clinical studies to define O-GlcNAcylation and its relationship with the anti-tumor effect in T cells.
4. Cancer Therapeutics Targeting O-GlcNAcylation
The role of hyper-O-GlcNAcylation in metastasis and resistance to chemotherapy in cancer as well as its potential role as a prognostic marker has prompted the development of targets directed at O-GlcNAcylation [
17]. Cell lines of breast, colorectal, prostate, and hepatocellular carcinoma treated with investigational OGT inhibitors have shown a significant decrease in tumor growth [
105,
106,
107,
108,
109].
Despite the promising pre-clinical data of OGT inhibitors, many hurdles remain, including the physiologic role of OGT involved in energy metabolism of normal cells [
3]. Agents targeting OGT directly, such as small molecules and bisubstrate inhibitors, were initially ideal potential therapeutic options in the treatment of cancers [
110]. However, the possibility of off-target toxicities for small molecules targeting OGT, and the inability to permeate through cells may render bisubstrate inhibitors ineffective [
111].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13215365