Flavonoids are one of the most diverse families of bioactive phytochemicals, with over 9000 different compounds. According to IUPAC Recommendations (2017), the term “flavonoid” refers to compounds that have the basic structure of phenyl-substituted propylbenzene derivatives with C15 skeleton, C16 skeleton, and flavonolignans with C6–C3 lignan precursors. Flavonoids are divided into six subclasses; isoflavones, flavones, flavanols, flavonols, flavanones, and anthocyanins, are abundant in plants and their metabolic routes have been thoroughly explored using biochemical and molecular approaches.
- flavone
- flavanone
- flavonol
- flavanol
- isoflavone
- anthocyanin
- reactive oxygen species
1. Introduction
Diabetes mellitus (DM) is one of the most deadly non-communicable diseases that leads to extensive impairments of organs and body functions. The increasing incidence of DM and its related complications have contributed to the surge of morbidity and mortality rate. DM affects about 463 million people aged between 20 to 79 years in 2019, and this figure is expected to climb up to 700 million by 2045 [1]. Moreover, DM is also one of the root causes for the development of cardiovascular diseases (CVD), which further exacerbate the mortality risk among patients with DM [2]. One of the complications resulting from chronic DM is diabetic cardiomyopathy (DCM). DCM is a cardiac pathological condition in patients with DM characterized by the appearance of aberrant myocardial morphology and cardiac functions in the truancy of other factors, such as coronary artery disease, hypertension, and prominent valvular disease [3]. Due to DCM, patients with DM are more likely to suffer from heart failure compared to their healthy counterparts [3]. This is why DCM is one of the most devastating consequences directly caused by DM.
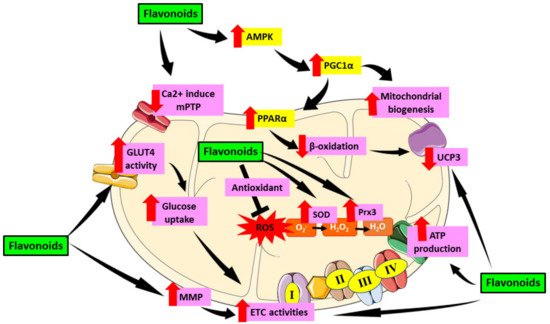
The heart has a high energy consumption in order to efficiently pump and supply blood throughout the body. Hence, it has a high density of mitochondria population to fuel its activities. However, this dependence exposes the heart to deleterious consequences when mitochondrial malfunction occurs. Mitochondria serve a critical part in oxygen metabolism, hence it is crucial to understand the effects of their dysfunction in patients suffering from metabolic disorders, particularly diabetes [4]. In DCM, the minimal glucose utilization will shift to fatty acid, leading to energetic inefficiency [5]. Since the mitochondria lost its efficiency in energy production, mitochondrial dysfunction will then follow. The role of mitochondrial dysfunction in the progression of DCM has been well established in earlier studies [6][7][8]. As the heart contains a high amount of mitochondria, cardiac mitochondrial dysfunction can lead to cardiac oxidative stress which aggravates the development of DCM. Indeed, the diabetic patients heart mitochondria are typically found to have deteriorated in number and structure, exhibiting increased reactive oxygen species (ROS) emission, and compromised mitochondrial respiratory capacity in the mitochondria [9]. Thus, treatment targeting mitochondrial-induced oxidative stress is very crucial in suppressing DCM.
For more than 40 years, the pathogenesis and mechanisms involved in DCM’s development and progression has been well-studied and documented as well as of its preventive measures and potential therapeutic agents. Despite this, effective remedies for preventing and treating DCM remains unclear [10]. The need of having a treatment for DCM is of utmost importance considering that there is no specific treatment targeting DCM up to the moment [11][12]. Presently, diabetes management is only based on combination of lifestyle modification and therapeutic medications to regulate blood glucose level through glucose-lowering agents or insulin replacement therapy as well as with management of cardiovascular complications [12][13].
2. Therapeutic Role of Flavonoid in Alleviating Mitochondrial Dysfunction-Induced Oxidative Stress in Diabetic Cardiomyopathy
2.1. Flavones
2.2. Isoflavones
2.3. Flavonol
2.4. Flavanol
2.5. Anthocyanins
2.6. Flavanones
| Flavonoid Subclass | Type | Study Design | Dose | Results | Reference |
|---|---|---|---|---|---|
| Anthocyanin | Protocatechuic acid | In vivo; T1DM Sprague-Dawley rats | 50 and 100 mg/kg/day | Reduce mitochondrial ROS levels, attenuated mitochondrial depolarization and decreased mitochondrial swelling in cardiomyocytes. | [50] |
| Flavones | Vitexin | In vitro; H9C2 cells | 1, 3, 10, and 30 µM | Improve mitochondrial ATP production Revive mitochondrial respiratory function by increasing expression of levels of COX IV and SDHB in H9c2 cells. | [61] |
| Rutin | In vivo; T2DM Wistar rats | 100 and 200 mg/kg/day | Improve co-enzyme Q9 and Q10 in the mitochondria. | [25] | |
| Luteolin | In vivo; T1DM Sprague-Dawley rats | 100 mg/kg/day | Increase MnSOD and eNOS expression and decrease Ca2+ induced mPTP opening and mitochondrial inner membrane in cardiomyocytes. | [23] | |
| Isoflavones | In vitro; H9C2 cells | 20–200 μg/mL | Reduce mitochondrial-induce oxidative by lowering mitochondrial ROS generation, depolarization of ΔΨm through SIRT-1 pathway or PPAR-α which further attenuate mitochondrial dysfunction and thus conserve cardiomyocytes health. | [31] | |
| In vivo; T1DM Sprague-Dawley rats | 25, 50, and 100 mg/kg orally |
Maintained the AMPK and SIRT1 levels. | [32] | ||
| Flavonol | Icariin | In vivo and in vitro; db/db, db/+ mice and C57 mice cardiomyocytes | 7.5, 15, and 30 µM | Upregulate myocardium gene apelin and the cardiac mitochondrial matrix gene Sirt3. Increase the mitochondrial membrane potential. Reduce mitochondria ROS production. |
[41] |
| Flavonol | Quercetin | In vivo and in vitro; T1DM Wistar rats and H9C2 cells | 50 mg/kg and 1 and 10 μM | Induce Prx-3 expression, causing downregulation in myocardial UCP3 protein. Reduce cardiac Trx-2 expression and TrxR2 activity. Induce the expression of transcription factor Nrf2/Nrf1. |
[42] |
| Quercetin and Kaempferol | In vivo; T1DM albino rats | 200 mg/kg/twice daily | Enhance the expression SOD1 gene, PGC 1α gene and ATpase and improve mitochondrial function. | [43] | |
| Taxifolin/dihydroquercetin | In vivo and in vitro; T1DM C57BL/6 mice and H9C2 cells | 10, 20, and 40 µg/mL and 25, 50, and 100mg/kg/day | Restore mitochondrial transmembrane potential in H9c2 cell lines. | [62] | |
| Dihydromyricetin | In vivo; T1DM C57BL/6 mice | 100 mg/kg/day | Enhance ATP levels, CS activity, and complex Ι/ΙΙ/ΙΙΙ/ΙV activities, increase ΔΨm. | [40] | |
| Flavanol | Epigallocatechin-3-gallate | In vivo; T2DM Goto–Kakizaki rats | 100 mg/kg/day | Revive Complex I, III, IV, and VDAC1 activities as well as recover mtDNA copies and the mitochondrial dehydrogenase activities. | [45] |
| Epicatechin | In vivo and in vitro; T2DM C57BL/6 mice and HCAEC cells | 100 nM and 1 mg/kg/day | Blocked the suppressive effect of high glucose on heart mitochondrial biogenesis involving mitofilin, SIRT1, PGC-1α, TFAM protein levels and reversed the high level of eNOS-O-GlcNAc of diabetic heart. | [46] | |
| Flavanone | Naringin | In vitro; H9C2 cells | 5 μM | Prevent the HG-induced loss in ΔΨm. | [56] |
| Naringin | In vivo and in vitro; T1DM Sprague-Dawley rats and H9C2 cells | 80 μM and 25, 50, and 100 mg/kg/day | Reduce the downregulation of KATP channels. | [57] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms222111616
References
- International Diabetes Federation (IDF). Diabetes Facts & Figures. 2019. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 30 June 2021).
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492.
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638.
- Cieluch, A.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Can we prevent mitochondrial dysfunction and diabetic cardiomyopathy in Type 1 diabetes mellitus? Pathophysiology and treatment options. Int. J. Mol. Sci. 2020, 21, 2852.
- Maack, C.; Lehrke, M.; Backs, J.; Heinzel, F.R.; Hulot, J.-S.; Marx, N.; Paulus, W.J.; Rossignol, P.; Taegtmeyer, H.; Bauersachs, J. Heart failure and diabetes: Metabolic alterations and therapeutic interventions: A state-of-the-art review from the Translational Research Committee of the Heart Failure Association–European Society of Cardiology. Eur. Heart J. 2018, 39, 4243–4254.
- Bingchao, Q.; Linjie, H.; Zhao, Y.; Zhang, L.; Yuanfang, H.; Li, J.; Congye, L.; Zhang, B.; Qichao, H.; Jinliang, X. Akap1 deficiency exacerbates diabetic cardiomyopathy in mice by NDUFS1-mediated mitochondrial dysfunction and apoptosis. Diabetologia 2020, 63, 1072–1087.
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N. Reduced reticulum–mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020, 115, 1–21.
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.-H. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation 2019, 139, 1913–1936.
- Katunga, L.A.; Gudimella, P.; Efird, J.T.; Abernathy, S.; Mattox, T.A.; Beatty, C.; Darden, T.M.; Thayne, K.A.; Alwair, H.; Kypson, A.P. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol. Metab. 2015, 4, 493–506.
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607.
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial dysfunction in diabetic cardiomyopathy: The possible therapeutic roles of phenolic acids. Int. J. Mol. Sci. 2020, 21, 6043.
- Tay, Y.; Bakar, M.; Azmi, M.; Saad, N.; Awang, K.; Litaudon, M.; Kassim, M. (Eds.) Inhibition of Carbohydrate Hydrolysing Enzymes, Antioxidant Activity and Polyphenolic Content of Beilschmiedia Species Extracts. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 716, p. 012007.
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front. Physiol. 2018, 9, 1514.
- Rauter, A.P.; Ennis, M.; Hellwich, K.-H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of flavonoids (IUPAC recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486.
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A.
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943.
- Sun, Y.; Qiao, L.; Shen, Y.; Jiang, P.; Chen, J.; Ye, X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 2013, 78, C37–C42.
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92.
- Jia, Q.; Wang, Y.; Liu, X.; Ma, S.; Yang, R. Effects of genistein on Nrf2/HO-1 pathway in myocardial tissues of diabetic rats. J. Cent. South Univ. Med. Sci. 2019, 44, 850–856.
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev. Res. 2019, 80, 294–309.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Yang, J.-T.; Qian, L.-B.; Zhang, F.-J.; Wang, J.; Ai, H.; Tang, L.-H.; Wang, H.-P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015, 65, 349–356.
- Zhang, B.; Chen, Y.; Shen, Q.; Liu, G.; Ye, J.; Sun, G.; Sun, X. Myricitrin attenuates high glucose-induced apoptosis through activating Akt-Nrf2 signaling in H9c2 cardiomyocytes. Molecules 2016, 21, 880.
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J. Transl. Med. 2015, 13, 1–14.
- Chirumbolo, S. Dietary assumption of plant polyphenols and prevention of allergy. Curr. Pharm. Des. 2014, 20, 811–839.
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222.
- Andor, B.; Danciu, C.; Alexa, E.; Zupko, I.; Hogea, E.; Cioca, A.; Coricovac, D.; Pinzaru, I.; Pătrașcu, J.M.; Mioc, M. Germinated and ungerminated seeds extract from two lupinus species: Biological compounds characterization and in vitro and in vivo evaluations. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–8.
- Ko, K.-P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010.
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.-A.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets 2018, 19, 841–853.
- Upadhyay, S.; Mantha, A.K.; Dhiman, M. Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. J. Ethnopharmacol. 2020, 258, 112690.
- Laddha, A.P.; Kulkarni, Y.A. Daidzein mitigates myocardial injury in streptozotocin-induced diabetes in rats. Life Sci. 2021, 284, 119664.
- Colareda, G.A.; Matera, S.I.; Bayley, M.; Ragone, M.I.; Flores, M.L.; Córdoba, O.L.; Consolini, A.E. Lepidium meyenii (maca) and soy isoflavones reduce cardiac stunning of ischemia-reperfusion in rats by mitochondrial mechanisms. J. Tradit. Complement. Med. 2021, 3, 4.
- Farruggio, S.; Raina, G.; Cocomazzi, G.; Librasi, C.; Mary, D.; Gentilli, S.; Grossini, E. Genistein improves viability, proliferation and mitochondrial function of cardiomyoblasts cultured in physiologic and peroxidative conditions. Int. J. Mol. Med. 2019, 44, 2298–2310.
- Zhao, Y.; Guo, R.; Li, L.; Li, S.; Fan, G.; Zhao, X.; Wang, Y. Tongmai formula improves cardiac function via regulating mitochondrial quality control in the myocardium with ischemia/reperfusion injury. Biomed. Pharmacother. 2020, 132, 110897.
- Iwashina, T. Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull. Natl. Mus. Nat. Sci. 2013, 39, 25–51.
- Rajagopalan, G.; Chandrasekaran, S.P.; Carani Venkatraman, A. Troxerutin attenuates diet-induced oxidative stress, impairment of mitochondrial biogenesis and respiratory chain complexes in mice heart. Clin. Exp. Pharmacol. Physiol. 2017, 44, 103–113.
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; De Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C.J. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine 2014, 21, 109–117.
- Zakaria, N.; Khalil, S.R.; Awad, A.; Khairy, G.M. Quercetin Reverses Altered Energy Metabolism in the Heart of Rats Receiving Adriamycin Chemotherapy. Cardiovasc. Toxicol. 2018, 18, 109–119.
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. BioMed Res. Int. 2017, 2017, 13.
- Ni, T.; Lin, N.; Huang, X.; Lu, W.; Sun, Z.; Zhang, J.; Lin, H.; Chi, J.; Guo, H. Icariin ameliorates diabetic cardiomyopathy through Apelin/Sirt3 Signalling to improve mitochondrial dysfunction. Front. Pharmacol. 2020, 11, 256.
- Arkat, S.; Umbarkar, P.; Singh, S.; Sitasawad, S.L. Mitochondrial peroxiredoxin-3 protects against hyperglycemia induced myocardial damage in diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 97, 489–500.
- Ahmed, E.; Ebrahim, H.S.E.D. Biochemical and Molecular Study on the Effect of Murraya Koenigii (Curry) and Moringa Oleifera on Cardiac Mitochondrial Dysfunction in Diabetic Rats. World J. Pharm. Pharm. Sci. 2018, 7, 1062–1076.
- Iglesias, D.E.; Bombicino, S.S.; Boveris, A.; Valdez, L.B. (+)-Catechin inhibits heart mitochondrial complex I and nitric oxide synthase: Functional consequences on membrane potential and hydrogen peroxide production. Food Funct. 2019, 10, 2528–2537.
- Liu, J.; Tang, Y.; Feng, Z.; Long, J. (–)-Epigallocatechin-3-gallate attenuated myocardial mitochondrial dysfunction and autophagy in diabetic Goto–Kakizaki rats. Free. Radic. Res. 2014, 48, 898–906.
- Ramírez-Sánchez, I.; Rodríguez, A.; Ulloa, A.M.; Ceballos, G.; Villarreal, F. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes Vasc. Dis. Res. 2016, 13, 201–210.
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908.
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90.
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017, 5, 1–9.
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23.
- Lim, Y.-C.; Budin, S.B.; Othman, F.; Latip, J.; Zainalabidin, S. Roselle polyphenols exert potent negative inotropic effects via modulation of intracellular calcium regulatory channels in isolated rat heart. Cardiovasc. Toxicol. 2017, 17, 251–259.
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244.
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Jayasingha, J.A.C.C.; Kim, G.-Y. Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF-κB-and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production. Oxidative Med. Cell. Longev. 2021, 2021, 17.
- Skemiene, K.; Liobikas, J.; Borutaite, V. Anthocyanins as substrates for mitochondrial complex I–protective effect against heart ischemic injury. FEBS J. 2015, 282, 963–971.
- Li, F.; Lang, F.; Wang, Y.; Zhai, C.; Zhang, C.; Zhang, L.; Hao, E. Cyanidin ameliorates endotoxin-induced myocardial toxicity by modulating inflammation and oxidative stress through mitochondria and other factors. Food Chem. Toxicol. 2018, 120, 104–111.
- Huang, H.; Wu, K.; You, Q.; Huang, R.; Li, S. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int. J. Mol. Med. 2013, 32, 396–402.
- You, Q.; Wu, Z.; Wu, B.; Liu, C.; Huang, R.; Yang, L.; Guo, R.; Wu, K.; Chen, J. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016, 230, 197–214.
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.-W.; Lee, J.H. Amelioration of high fructose-induced cardiac hypertrophy by naringin. Sci. Rep. 2018, 8, 1–11.
- Salehcheh, M.; Alboghobeish, S.; Dehghani, M.A.; Zeidooni, L. Multi-walled carbon nanotubes induce oxidative stress, apoptosis, and dysfunction in isolated rat heart mitochondria: Protective effect of naringin. Environ. Sci. Pollut. Res. 2020, 27, 13447–13456.
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552.
- Xue, W.; Wang, X.; Tang, H.; Sun, F.; Zhu, H.; Huang, D.; Dong, L. Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomed. Pharmacother. 2020, 124, 109849.
- Sun, X.; Chen, R.-C.; Yang, Z.-H.; Sun, G.-B.; Wang, M.; Ma, X.-J.; Yang, L.-J.; Sun, X.-B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232.
