Chilli (Capsicum annuum L.) is an herbaceous crop and plays an important role as common spices and vegetables. Pepper (Capsicum spp.) is one of the most cost-effective and agricultural vegetables in the world.
- L.
- varietal improvement
- heterosis
- breeding techniques
1. Introduction
Due to the scanty local production and yield, a large number of countries are to import Chilli from other countries. Improvement of high-yielding varieties is one of the main considerations to increase production. Quite possibly the main component for extending of production area is the prerequisite of high-yielding assortments. Consequently, several breeding projects have been carried out to increase the production of the crop [1][2][3]. To increase the current productivity of chilli as a vegetable crop less consideration has been paid to hereditary improvement. Utilizing the indigenous inferior quality cultivars with diminished resistance of open-pollinated genotypes against biotic stresses, are liable for low profitability [4]. To stay away from hereditary disintegration, it is essential to utilize existing genetic materials, the profitability chilli couldn’t be earned by utilizing open-pollinated varieties. Identification, and utilization, of appropriate parental lines, are fundamental for developing possible hybrids of chilli with high and stable yield. Moreover, improving yield, quality, and tolerance to cope with biotic and abiotic stress at the same time is a critical need of time [5].
In a hybrid development strategy, genetic diversity linked with combining ability is a criterion for selecting parents. At the point of crossing intersection among highly related parents then a low increase of heterosis is noticed, however high heterosis happens when the cross is made between parents of greater dissimilarity [3] and [1]. In the advance line selection process, heterotic group identification, pattern identification, and analysis of combining ability of sets of inbred lines play a significant role in chilli breeding [2] and [1]. At the same time, many studies have been conducted using heterosis breeding to increase chilli yield and quality traits [1][6][7].
Diallel research may be used successfully to learn about the combining ability of a set arrangement of self-and cross-pollinated populations. To distinguish appropriate parents for utilizing in crossing programs, including the assessment of the parents for a progression of crosses, and for estimation of heterosis in chilli, should be possible effectively through diallel technique [4][7]. Heterosis breeding employing the diallel technique can guarantee an improvement in chilli productivity due to the existence of integral quality activities and the occurrence of a high degree of non-additives quality effects [8][9][10]. Essentially, the system of peppers raiser is to accumulate in specific qualities a solitary cultivar with higher hereditary possible such as productivity, disease resistance, and bioactive compounds.
2. General Purposes and Classical Breeding Techniques Used for Capsicum Improvement
2.1. Common Goal for Capsicum Breeding
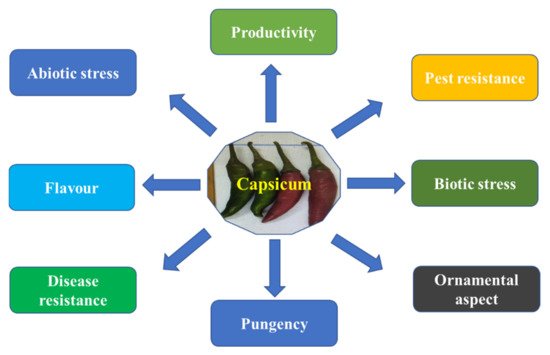
2.2. Classical Breeding Techniques Used for Chilli Improvement
| Name of the Approaches | Assumption | Reference |
|---|---|---|
| Mass selection | The next growing year stored seeds of the finest plants; the oldest technique | [24] |
| Pedigree method | Maintaining matings records and progenies. This comprises the selection and self-pollination of single plants. | [25] |
| SSD (Single seed descent) | This approach involves advances without selection of generations and is also used for the production of recombinant inbred lines. | [18] |
| Recurrent selection | Keep choosing individuals from a population and then crossing across to establish a new population. | [26] |
| Backcross | Especially for characteristics regulated by one or few genes involving the selection of individual plants and subsequent crossings for recurring parents | [19] |
| Hybridization | From one species genes or variations migrate via the crossover process | [24] |
2.2.1. Mass Selection
2.2.2. The Pedigree Method
2.2.3. Single Seed Descent Method
2.2.4. Recurrent Selection
2.2.5. Backcross
2.2.6. Hybridization
2.2.7. Genetic Basis of Hybridization

2.3. Using the Male Sterile Lines
This entry is adapted from the peer-reviewed paper 10.3390/agronomy11112217
References
- Bhutia, N.D.; Seth, T.; Shende, V.D.; Dutta, S.; Chattopadhyay, A. Estimation of heterosis, dominance effect and genetic control of fresh fruit yield, quality and leaf curl disease severity traits of chilli pepper (Capsicum annuum L.). Sci. Hortic. 2015, 182, 47–55.
- Nagaraju, M.M.; Kumary, I.S.; Celine, V.A.; Devi, C.R.S.; Manju, P. Development of F1 Hybrids in Chilli (Capsicum annuum L.) for Dual Purpose (Green as well as Dry). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 84–96.
- Rohini, N.; Lakshmanan, V. Evaluation studies of hot pepper hybrids (Capsicum annuum L.) for yield and quality characters. Electron. J. Plant Breed. 2017, 8, 643–651.
- Herath, H.M.S.N.; Rafii, M.Y.; Ismail, S.I.; Nakasha, J.J.; Ramlee, S.I. Improvement of important economic traits in chilli through heterosis breeding: A review. J. Hortic. Sci. Biotechnol. 2021, 96, 14–23.
- Sreenivas, M.; Sharangi, A.B.; Banerjee, S.; Kumar Maurya, P.; Bhattacharjee, T. Selecting Parental Lines among Genotypes of Capsicum annuum for Hybridization Aiming at Dry Fruit Yield Improvement. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1881–1899.
- Alok, C.; Rajesh, K.; Solankey, S.S. Estimation of heterosis for yield and quality components in chilli (Capsicum annuum L.). Afr. J. Biotechnol. 2013, 12, 6605–6610.
- Singh, D.K.; Pramod, T.; Jain, S.K. Heterosis studies for growth, flowering, and yield of chilli (Capsicum annuum L.). Pantnagar J. Res. 2012, 10, 61–65.
- Ganefianti, D.W.; Fahrurrozi, F. Heterosis and Combining Ability in Complete Diallel Cross of Seven Chili Pepper. AGRIVITA J. Agric. Sci. 2018, 40, 360–370.
- Hasanuzzaman, M.; Golam, F. Gene actions involved in yield and yield contributing traits of chilli (Capsicum annuum L.). Aust. J. Crop Sci. 2011, 5, 1868–1875.
- Do Rêgo, E.R.; do Rêgo, M.M.; Finger, F.L.; Cruz, C.D.; Casali, V.W.D. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica 2009, 168, 275–287.
- Chiou, K.L.; Hastorf, C.A.; Bonavia, D.; Dillehay, T.D. Documenting Cultural Selection Pressure Changes on Chile Pepper (Capsicum baccatum L.) Seed Size Through Time in Coastal Peru (7600 B.P.–Present). Econ. Bot. 2014, 68, 190–202.
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; Sánchez García, Y.; Jarret, R.; Ehrendorfer, F. The evolution of chili peppers (Capsicum-Solanaceae): A cytogenetic perspective. In Proceedings of the VI International Solanaceae Conference: Genomics Meets Biodiversity, Madison, WI, USA, 23–27 July 2016; pp. 137–170.
- Hoffmann, A.M.; Noga, G.; Hunsche, M. Acclimations to light quality on plant and leaf level affect the vulnerability of pepper (Capsicum annuum L.) to water deficit. J. Plant Res. 2015, 128, 295–306.
- Chhapekar, S.; Kehie, M.; Ramchiary, N. Advances in Molecular Breeding of Capsicum Species. Biotechnological Tools for Genetic Resources; Daya Publishing House: Darya Ganj, India, 2016; pp. 397–419.
- Baruah, S.; Zaman, M.K.; Rajbongshi, P.; Das, S. A Review on recent researches on Bhutjolokia and pharmacological activity of capsaicin. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 89–94.
- Padilha, H.K.M.; Pereira, E.D.S.; Munhoz, P.C.; Vizzotto, M.; Valgas, R.A.; Barbieri, R.L. Genetic variability for synthesis of bioactive compounds in peppers (Capsicum annuum) from Brazil. Food Sci. Technol. 2015, 35, 516–523.
- Kim, S.; Park, M.; Yeom, S.-I.; Kim, Y.-M.; Lee, J.M.; Lee, H.-A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278.
- Ulhoa, A.B.; Pereira, T.N.; Silva, R.N.; Ragassi, C.F.; Rodrigues, R.; Pereira, M.G.; Reifschneider, F.J.B. Caracterização molecular de linhagens de pimenta do tipo Jalapeño amarelo. Hortic. Bras. 2014, 32, 35–40.
- Udaya Prakash, N.K.; Bhuvaneswari, S.; Sripriya, N.; Prameela, L.; Bhagya, R.; Radhika, B.; Balamurugan, A.; Arokiyaraj, S. Antioxidant activity of common plants of Northern Tamil Nadu, India. Int. J. Pharm. Pharm. Sci. 2014, 6, 128–132.
- Negi, R.; Thakur, S.; Sharma, P. Advances in the Breeding of Bell Pepper—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2272–2281.
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140.
- Norman, A.; Taylor, J.; Edwards, J.; Kuchel, H. Optimising genomic selection in wheat: Effect of marker density, population size and population structure on prediction accuracy. G3 Genes Genomes Genet. 2018, 8, 2889–2899.
- Rao, A.M.; Anilkumar, C. Conventional and Contemporary Approaches to Enhance Efficiency in Breeding Chilli/Hot Pepper. In Accelerated Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 2, pp. 223–269.
- Chen, J.; Luo, M.; Li, S.; Tao, M.; Ye, X.; Duan, W.; Zhang, C.; Qin, Q.; Xiao, J.; Liu, S. A comparative study of distant hybridization in plants and animals. Sci. China Life Sci. 2018, 61, 285–309.
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in plants: Old ideas, new techniques. Plant Physiol. 2017, 173, 65–78.
- Rodrigues, R.; Gonçalves, L.S.; Bento, C.d.S.; Sudré, C.P.; Robaina, R.R.; do Amaral, A.T., Jr. Combining ability and heterosis for agronomic traits in chili pepper. Hortic. Bras. 2012, 30, 226–233.
- Sthapit, B.; Shrestha, P.; Subedi, M.; Castillo-Gonzales, F. Mass selection: A low-cost, widely applicable method for local crop improvement in Nepal and Mexico. In Participatory Approaches to the Con-Servation and Use of Plant Genetic Resources; Friis-Hansen, E., Sthapit, B.R., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 2000; p. 111.
- Gosal, S.S.; Pathak, D.; Wani, S.H.; Vij, S.; Pathak, M. Accelerated Breeding of Plants: Methods and Applications. In Accelerated Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 1, pp. 1–29.
- De Sá Mendes, N.; Santos, M.C.P.; Santos, M.C.B.; Cameron, L.C.; Ferreira, M.S.L.; Gonçalves, É.C.B.A. Characterization of pepper (Capsicum baccatum)—A potential functional ingredient. LWT 2019, 112, 108209.
- Visalakshi, M.; Pandiyan, M. Crop improvement in chillies: An overview. Int. J. Chem. Stud. 2018, 6, 1736–1744.
- Bermejo, C.; Gatti, I.; Cointry, E. In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 585–590.
- Barroso, P.A.; Rêgo, M.M.D.; Crispim, J.G.; Costa, M.D.P.S.D.; Rêgo, E.R.D. How to shorten a plant breeding program? A case study with ornamental peppers. Crop. Breed. Appl. Biotechnol. 2019, 19, 193–199.
- Sarath Babu, B.; Pandravada, S.R.; Prasada Rao, R.D.V.J.; Anitha, K.; Chakrabarty, S.K.; Varaprasad, K.S. Global sources of pepper genetic resources against arthropods, nematodes and pathogens. Crop Prot. 2011, 30, 389–400.
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Mohammad Yusoff, M.; Miah, G.; Usman, M. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122.
- Bosland, P.W.; Votava, E.J.; Votava, E.M. Peppers: Vegetable and Spice Capsicums; CABI: Wallingford, London, UK, 2012.
- Do Rego, E.R.; do Rêgo, M.M.; Finger, F.L. Production and Breeding of Chilli Peppers (Capsicum spp.); Springer International Publishing: Cham, Switzerland, 2016; pp. 57–80.
