Monoclonal antibodies (mAbs) are an important class of biotherapeutics. MAbs, like any globular protein, are amphiphilic and readily adsorb to interfaces, potentially causing structural deformation and even unfolding. Desorption of structurally perturbed mAbs is often hypothesized to promote aggregation, potentially leading to the formation of subvisible particles and visible precipitates. Since mAbs are exposed to numerous interfaces during biomanufacturing, storage and administration, many studies have examined mAb adsorption to different interfaces under various mitigation strategies.
1. Why is Studying Monoclonal Antibody Adsorption Important?
Biopharmaceuticals are highly versatile and over the past ten years or so, bioengineered proteins, especially monoclonal antibodies (mAbs), have emerged as important drugs in treating several major disease areas, including immune checkpoint inhibitors (immuno-oncology) and treatments for chronic diseases [
1]. The industrial process to produce therapeutic mAbs can remove aggregation and other impurities during the downstream process but problems can be encountered when there are incompatibilities with interfaces during fill-finish, transportation, storage and administration. Interactions with interfaces can result in denaturation of the antibody and potential sub-visible particle formation [
2,
3,
4,
5,
6]. The design of mAbs rarely involves considerations for the effects of interfacial adsorption, to this end, products can sometimes face delays when incompatibilities between mAb and interfaces result in internal specification failures. Such failures can result in product batches being discarded, which is expensive and can delay important treatments to market. Studying mAb adsorption at interfaces where incompatibilities are detrimental to production could provide an insight into what is triggering unwanted aggregation, and this knowledge could be used to guide how therapeutics are formulated/designed to help prevent batch failures caused by material incompatibilities and related project delays.
The therapeutic protein market has been steadily gaining traction [
7] and in 2017 the global therapeutic monoclonal antibody (mAb) market was worth approximately USD 108 billion with an expected generated revenue by the end of 2023 around USD 219 billion [
8]. The majority of current mAb therapeutics are derived from natural IgG1 proteins, with sequence modifications in Fab or Fc or both. In spite of extensive efforts made in their development, there is no assurance about their stability under different stages of production and application. Lack of structural stability can cause a range of issues as mAbs at high concentrations (> 100 mg/mL) tend to aggregate, triggering the growth of larger aggregates and possible precipitates. Protein adsorption and desorption constitute a crucial step that may induce structural instability and promote aggregate nucleation and growth mechanisms. Given the fast expansion in the development of protein drugs, a good way to reduce risk when developing products is by considering adsorption before challenges emerge in late-stage development. Following the strict rules imposed by regulatory filing and review, a protein drug must remain stable over its stated lifetime, contain no more than 6000 sub-visible particles ≥ 10 µm, 600 sub-visible particles ≥ 25 µm per container, as well as harbour practically no visible particulates [
9].
2. Biopharmaceutical Production
This section will introduce the stages in producing biopharmaceuticals and highlight when interfacial adsorption can become problematic.
2.1. Upstream Process
Initially, in biopharmaceutical production, a stock culture is prepared with cells able to produce recombinant mAb. After optimizing parameters such as temperature, pH and the type of process, large scale bioreactors are used to acquire large quantities of the desired mAb. Separation of mAbs as target proteins from the rest of cell masses can be achieved by precipitation, centrifugation and porous membrane filtration. Most porous membranes used in different types of filtration are fabricated from polymeric materials, with pore sizes ranging from micro to nanometre range. Separation is largely based on effective pore sizes. Interactions between mAb and pore surface via entropic, hydrophobic and electrostatic effects influence how a given mAb adsorbs and how adsorbed mAb layer inside the pore surface mediates mAb permeation and subsequent blockage or filter fouling [
10].
2.2. Downstream Process
At the present stage, mAb purification is often carried out by different forms of liquid chromatography wherein the clarified cell media is first passed through a packed porous resin derivatized by protein A to capture the Fc, followed by orthogonal purification processes utilizing different protein surface charge (mAbs typically have a pI > 7.5) to further remove host cell protein and a final polishing step [
11,
12,
13,
14]. Like porous membrane separation, these column-based separations also expose the mAb to very high surface areas that are potentially destabilizing as a consequence of adsorption/desorption. Viral clearance is required to reduce the risk of known, or unknown, viral contaminants. There are several methods used to achieve this including low pH treatments, detergent inactivation viral filtration as well as viral clearance steps such as protein A chromatography [
10,
15].
Highly purified proteins are often unstable in solution because they have been removed from their native environments (e.g., serum or cytosol) which supported their native structures. In addition, many mAbs are engineered, containing large segments of artificially designed sequences (e.g., scFv) that may not form well folded structures. Formulation is a process that improves mAb stability by adding buffer/salt ions, amino acids, polyols and non-ionic surfactants [
16]. The mAb drug substance will be filled into bulk plastic containers and may be frozen for long term storage.
2.3. Fill/Finish
The drug substance is shipped to the fill site and thawed if it was frozen. The drug substance is generally temperature equilibrated, pooled, mixed and sterile-filtered before being filled into glass vials, pre-filled syringes or related devices with a target shelf-life of greater than 18 months. Adsorption and desorption at solid/water and air/water interface can negatively impact product quality.
Shaking and shearing of a liquid product in transit are inevitable and will cause the mAb to be adsorbed/desorbed at the expanding/contracting air/liquid interface, respectively, also potentially leading to aggregation in solution [
17]. Thus, apart from adsorption and desorption onto different substrate surfaces, the process at the air/water interface can also underline mAb stability.
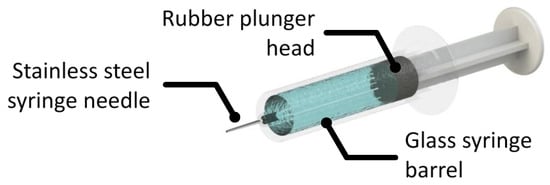
Finally, it is useful to comment on primary containers. As parenteral administration is often necessary, tremendous effort has been put on improving administrative efficiency, reliability and convenience. The development of prefilled syringes helps overcome the lack of convenience, accuracy and some safety concerns that come with traditional parenteral administration. The interfaces present in prefilled syringes are presented in Figure 1. There can also be residual tungsten from the tip forming process. The syringe needle is generally stainless steel and many syringe barrels are made of glass or plastics (polyolefins). To ensure suitable break loose and glide forces, prefilled syringe plunger heads and the syringe barrel are often coated in silicone oil for lubrication. This approach has been incorporated into the formal surface preparation protocols for vial stoppers and syringes used for mAb storage and administration.
Figure 1. An illustration of a basic syringe system with a stainless steel needle, a glass barrel and a lubricated rubber plunger.
For products administered in the clinic, closed system transfer devices and IV bags can pose further challenges with regard to product-surface incompatibilities. Correct formulation of the final mAb product in an aqueous solution is important to prevent-product adsorption and sub-visible or visible particle formation [
18,
19,
20].
3. Introduction to Proteins, Monoclonal Antibodies and Adsorption
Proteins are polymers of amino acids that fold into well-defined local constructs such as helical strands, sheets and turns and three-dimensional (3D) structures. Structural folds are complex but proteins in their native states usually have well-defined 3D, or tertiary, structures with hydrophobic amino acids buried inside and polar and charged amino acids projected outside. Because of the anisotropic distribution of charged, polar, and apolar groups, some parts of a protein’s surface are more hydrophobic than other parts. These amphiphilic molecules spontaneously adsorb when exposed to interfaces. Fouling of surgical equipment, spoiling of contact lenses and contamination in food process plants [
21,
22,
23,
24,
25,
26,
27] represent examples where protein adsorption imposes a burden to technological processes. On the other hand, protein adsorption can be beneficial as well as hindering. For example, the fabrication of functional proteins onto detection surfaces of sensors and surfaces of implants is crucial in ensuring product reliability. Various technical processes must be deployed to either promote protein adsorption where desired or inhibit protein adsorption where undesired, but in all cases, the instability of proteins, often induced by adsorption/desorption processes, causes complications and adds uncertainty in either removing fouled proteins in the former or sustaining in protein bioactivities in the latter. IgG antibodies are proteins that provide the body immunity against pathogens. They are comprised of 4 peptide chains and come in 4 subclasses with differences lying in their location and number of interchain-disulphide bonds and their main role in the body is to initiate phagocytosis. Initiated mechanisms include complement-dependant cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and receptor blocking. CDC can spontaneously identify pathogens, the classical pathway is initiated by the binding of the antibody Fc to C1q protein. This results in a membrane attack complex and target cell lysis [
28]. ADCC is a cell-mediated immune response whereby a target cell is actively lysed. Antibodies bind to antigens on the target cell surface, then most commonly a natural killer cell’s Fc receptor (CD16) will bind to the Fc region of the antibody resulting in lysing of the unwanted cell [
29]. Blocking antibodies prevents other antibodies from binding to an antigen. One such mAb, Ipilimumab, works by reducing the downregulation of the immune system, thereby activating the immune system [
30].
4. Recent Advances in Adsorption Studies of Bioengineered mAbs at Different Interfaces
With mAbs now established as important therapeutic drugs, it is imperative to understand how to control their adsorption during manufacture to ensure a stable drug product [
66]. As discussed, it is hypothesized that mAbs, exposed to siliconized glass or plastic surfaces of a primary container, may adsorb/desorb and so aggregate through structural change.
4.1. The Air/Water Interface
An interface commonly encountered in biopharmaceutical production and storage, so it is important to study interfacial adsorption and desorption of mAbs. Unfortunately, it is difficult to gain basic information such as the changes of the adsorbed amount and layer thickness with time. We have studied the adsorption of COE-3 at the air/liquid interface using neutron reflection and surface tension experiments under different buffer conditions (mAb concentrations, pH and ionic strengths) [
67]. We have also isolated and investigated the adsorption of the Fc and Fab segments of COE-3 [
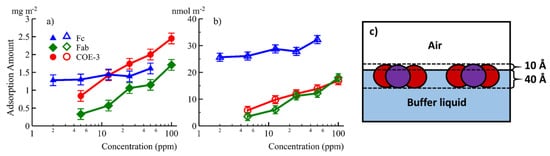
35]. The results implied that Fc and Fab have different interfacial properties, as shown in
Figure 2a,b. Fab dominated the concentration-dependent mAb adsorption while both Fab and Fc contributed to the amount of mAb adsorbed. Using multiple contrasts (NRW, Contrast-Match-Antibody water (CM2.58) and D
2O), the adsorbed layer structure and its immersion depth was revealed, as shown in
Figure 2c. Our neutron reflection study has shown that NRW and CM2.58 could offer significant benefits in emphasising mAb and mAb fragments (e.g., Fab and Fc) at the interface by showing the entire adsorbed layer and the region out of the water surface, respectively. The Fc layer remained constant at 40 Å while Fab and mAb layers increased very little from 45 to 50 Å when mAb concentration increased from 5 to 50 ppm. On the other hand, the volume fraction of the adsorbed antibody increased from 40% to 56%, which results in quite a drastic change in adsorbed amount, as can be seen in
Figure 2. These studies implied that the adsorbed mAb, Fc, and Fab all retained their globular structures and were oriented with their short axial lengths perpendicular to the air/liquid interface. The same measurement in D
2O revealed how the adsorbed protein layers mixed with water. The results showed that for COE-3, its Fab and Fc were predominantly immersed in water once adsorbed.
Figure 2. The equilibrated amount of adsorption in mg/m2 (a) and nmol/m2 (b) plotted against concentration for Fc (▲), Fab (♦) and the whole mAb COE-3 (●). (c) A schematic representations of the surface adsorbed COE-3 layers, Fab represented in red and Fc in purple. Reproduced with permission from Z. Li, ACS Applied Materials & Interfaces, 2017.
4.2. The SiO2/Water Interface
Native SiO2 formed on silicon makes for a good model to understand protein behaviour at glass/water interfaces due to the similarity to a glass surface. Because many natural proteins adsorb onto silica, it can be expected that bioengineered mAbs will adsorb as well but the difficulty lies in predicting the exact adsorbed amount, layer structure and conformational orientation etc. With many intertwined parameters, current studies can only be limited to quite narrow measurement ranges with a limited prediction power.
Type I borosilicate glass vials and pre-filled syringes are widely used in the storage of protein biopharmaceuticals. It is thus useful to understand how bioengineered proteins like mAbs adsorb at the glass/water interface to understand how adsorption can impact protein stability. Silica has been widely used as a model for glass to facilitate many protein adsorption studies by different techniques. Techniques such as spectroscopic ellipsometry and neutron reflection benefit greatly from an incredibly flat and reflective surface [
2,
48]. It is common to deploy several different techniques in a given study to try to obtain complementary information. While spectroscopic ellipsometry can measure how the adsorbed amount changes with respect to bulk mAb concentration, pH, ionic strength and time, AFM reveals the structure of adsorbed molecules and interfacial Fourier transform infrared spectroscopy reveals the secondary structure changes at the same interface.
One area of interest is to design coatings that can manipulate protein adsorption. Couston et al. investigated the adsorption of a mAb onto a silanized silica with octadecyltrichlorosilane (OTS) to generate octadecyl monolayer [
73]. This is a mimic of a hydrophobic plastic surface and they showed that adsorption was greatly reduced at the hydrophobic surface with only 2 mg/m
2 of mAb adsorbed, compared to around 5.5 mg/m
2 on the bare SiO
2 surface It was also shown that standard PS 80 could displace roughly 50% of the adsorbed protein on the hydrophobic surface due to an interaction between the fatty acid tail of the polysorbate and the modified surface. Benefits from coatings need to be compared with the risks of introducing potential leachables which if not properly handled can be a safety concern [
74].
4.3. The Stainless Steel/Water Interface
Interest in this interface stems from its practical relevance. It has been suggested that stainless steel can induce structural damage to adsorbed mAbs leading to aggregates in the bulk protein solution. Structural changes have been observed from the work of lysozyme or bovine serum albumin (BSA) adsorbed to different types of stainless steel as well as therapeutic fusion proteins [
43,
80]. These studies have also shown that adsorbed proteins could compromise the corrosion resistance of the steel surface and enhance metal ion release (up to 40-fold increase for BSA). These phenomena could potentially be alleviated if there were either a way to prevent protein adsorption or even make it occur in a more controlled manner. Furthermore, stainless steel has been observed to mediate protein aggregation. Stainless steel piston pumps we seen to produce particle formation on a novel fusion protein whereas this was not the case when a ceramic piston pump was involved [
43].
Using the NISTmAb (an IgG1 mAb reference material from the National Institute of Standard and Technology), Kalonia et al. [
81] revealed that the amount of subvisible particles (SVP) induced by stainless steel was substantially more than from alumina. SVPs tended to form more easily under extreme shear stresses. These authors also found that as mAb concentration increased SVPs were easier to produce. Intriguingly, only a small fraction of desorbed mAbs shed into solution could induce substantial SVP production. Neutron reflection helped reveal a high amount of the mAb deposited at the stainless steel interface over a wide concentration range. Shear stress could remove adsorbed mAbs leading to the formation of subvisible particles. The NR and quartz crystal microbalance studies also revealed that over a short time scale low and high mAb concentrations led to similar adsorption. Over a long time period, the adsorption at high concentrations continued whilst adsorption at low concentrations plateaued. Other studies have highlighted similar issues with material incompatibility [
82]. Bee et al. suggest that storage of biological products over a two year period would result in a small loss in protein mass but could still result in subvisible particles that could compromise the safety of the biotherapeutic if not handled correctly.
Modifying steel surfaces could be one way of tackling the unwanted phenomenon associated with protein adsorption to stainless steel surfaces. It has been shown that coating a polycaprolactone film onto 316L stainless steel can effectively reduce the amount of adsorbed BSA as determined via a BCA protein assay [
83]. Surface modifications could provide an effective way of limiting adsorption and potentially alleviate some undesirable repercussions of protein adsorption onto stainless steel. As with any coating, the risk of introducing leachables become a concerning issue [
74].
4.4. The Oil/Water Interface
Relevant to the use of silicone oil as a lubricating film for pre-filled syringes as outlined previously. Neutron reflection has been used to study protein adsorption at the oil/liquid interface [
84]. The technique involves a substrate such as quartz or sapphire to be used as a vehicle to create a stable oil/water interface. To reduce attenuation of the neutron beam the oil should be a thin layer, typically around 1–2 µm, but the challenge lies in keeping the structure of the film stable during the entire experiment. As explained previously, oil contrast matched to the solid substrate must be used to avoid a large signal from the oil/solid interface. Polydimethylsilance (PDMS) oil has been widely developed for lubricating the mAb pre-filled syringes, but hexadecane is used as a model oil at this stage to help develop the experimental capability, as it is more stable at the interface. Changes in water contrasts help highlight the adsorbed mAb layer differently, resulting in information about the adsorbed amount, mAb layer thickness, its extent of mixing with oil and water. Zarbakhsh et al. [
84] demonstrated the ability to resolve interfacial structures at the oil/water interface using neutron reflectometry with further work by Campana et al. [
85] investigating the adsorption of BSA at the water/hexadecane interface. Results for BSA adsorption showed that the protein formed a relatively thick (75 Å) layer in the oil phase, suggesting a hydrophobic region of the protein freely to interact with the oil resulting in changes in secondary and tertiary structures [
85].
This entry is adapted from the peer-reviewed paper 10.3390/molecules25092047