Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Role of molecules in in Andrology.
Hormones and cytokines are known to regulate cellular functions in the testes in various level such as spermatogenesis, modulation of cell junction restructuring between Sertoli cells and germ cells in the seminiferous epithelium. Cytokines and androgens are closely related, and both correct testicular development and the maintenance of spermatogenesis depend on their function. Cytokines also play a crucial role in the immune testicular system. The purpose of this papar is to explain the molecular mechanisms essentials for testicular and sexual function.
- Hormonal Correlation
- Molecular
- Andrology
- Fertility
1. Introduction
Male sexual function, from fertility to erectile function, requires a pattern of biochemical mechanisms connected to each other. A variety of molecules, such as cytokines, hormones, and immunomodulators, play a key role in maintaining homeostasis and a functional system.
Male fertility depends on the efficiency of a successful perpetuation of spermatogenesis, which is a highly organized process of germ cell differentiation in the seminiferous tubules. Spermatogonia stem cells (SSCs) are a subset of undifferentiated spermatogonia that are capable of self-renewal to maintain the pool of SSCs or to differentiate in spermatozoa [1].
Some of the most important aspects of normal testis development and function are modulated or driven by cytokine activities. Recent research findings suggest that the immune cell-associated cytokines are essential for fertility and to maintain testicular homeostasis. Cytokines are key mediators of immune cell function, and they are tightly regulated in the testes in order to protect and allow sperm production. It is also recognized that cytokines from non-immune cells are essential for normal adult testicular functions [2].
Cytokines and androgen action are closely related. The process of spermatogenesis is highly dependent on autocrine and paracrine communication among testicular cell types and the disruption of the androgen receptor (AR), a member of the nuclear receptor superfamily. New discoveries demonstrated the necessity of AR signaling for both external and internal male phenotype development. In fact, androgens are able to determine the expression of the male phenotype, including the outward development of secondary sex characteristics as well as the initiation and maintenance of spermatogenesis [3].
Growing evidence shows that miRNAs are specifically expressed in certain types of male germ cells, while others are universally expressed among different types of cells in the testes, and this evidence has shown that miRNAs are essential for male germ cell development and differentiation [4].
2. The Testis
It is well known that male genital tract inflammations are relevant co-factors in human subfertility and infertility [5]. Both infectious (viral and bacterial infections) and autoimmune diseases are commonly found in the testicular biopsies of patients with chronic inflammation of known or unknown etiology associated with infertility [6]. Lymphocyte infiltrations mimicking autoimmune orchitis may be found in the testicular biopsies of patients with other pathologies involving tissue damage and spermatic antigen release, such as testis trauma, cryptorchidia, and testicular cancer in situ [7].
The major functions of the testis are spermatogenesis and steroidogenesis. The latter is accomplished by Leydig cells localized in the interstitium as compact cell clusters closely associated with blood vessels. The connective tissue of collagen fibers, fibroblasts, and mesenchymal cells constitutes the interstitial tissue, which also contains cells of the immune system involved in innate and adaptive immune responses: macrophages and scarce dendritic cells; T and more rarely B lymphocytes; mast cells; and natural killer (NK) cells. Spermatogenesis occurs within the seminiferous tubules (STs), where Sertoli cells, targets of both testosterone and FSH, play a crucial role in germ cell (GC) proliferation and differentiation. These somatic cells, via specialized cell junctions, create the blood–testis barrier (BTB), which divides the ST into a basal and an adluminal compartment [8].
Gap junctions are composed of transmembrane proteins called connexins, which allow molecules smaller than 1 kDa to pass between the cytoplasmic compartments of two adjacent cells [9]. Tight junctions are regions where the outer leaflets of opposing Sertoli cell membranes come into contact, completely occluding intercellular space [10].
For completion of spermatogenesis, spermatocytes must migrate from the basal to the adluminal compartment of the seminiferous epithelium, crossing the BTB. This process requires that the Sertoli–Sertoli and Sertoli–GC junctions are disassembled and reassembled [11].
During the spermatogenesis process, hormones (testosterone, estrogens, and FSH) [12], cytokines (such as interleukin 1-α (IL-1α)), transforming growth factor-β3 (TGF-β3), tumor necrosis factor-α (TNF-α) [13], growth factors (such as hepatocyte growth factor) [14], and nitric oxide (NO) [15] all play a crucial role in the extremely tight regulation of the cell junction in the testis.
Many factors, such as interleukins and TNF, control immune cell function within the testis, and they are produced by “non-immune cells” to stimulate and maintain spermatogenesis; moreover, testicular Sertoli cells are found to produce interleukin-1 (IL1) [16].
Important aspects of normal testis development and function are modulated or driven by cytokine activities. Cytokines are key mediators of immune cell function, and the testis is a tissue in which cytokine functions are tightly regulated to protect and allow sperm production. Both Sertoli and Leydig cells can be stimulated to produce large amounts of the immunoregulatory cytokine, IL6, driven at least in part by endogenous IL1. Furthermore, IL1 and IL6 can regulate Sertoli cell and spermatogenic cell development [2]. TNF has dual actions as a signaling molecule regulating Sertoli cell function and cell death in response to toxic insults, and this activity is largely determined by the receptor with which it interacts [17].
Maintenance of the testicular environment for the production of sperm is dependent on cytokines secreted by both testicular somatic cells (Sertoli, Leydig, and peritubular cells) and resident immune cells. Some typically “protective” cytokines may, under pathological conditions, negatively impact testis function and physiology. In this regard, several studies show how some immune cell types are abundant in testicular tumors, but their characteristics in the testis cancer microenvironment remain poorly understood.
T cells and macrophages are typical cellular components of testicular tumors [18]. Under certain conditions, nascent tumor cells escape regulation by the host immune system by inducing APC malfunction, thus hijacking immune surveillance and, in turn, initiating immunosuppressive cell recruitment to create a tumor-tolerant microenvironment. High numbers of CD11c+ myeloid DC (mDC) were found in tumor tissues compared to healthy controls, with a proportion of mDC presenting an immature phenotype, potentially associated with cancer progression [19]. Fundamental knowledge regarding cytokines and molecular synthesis, target cells, and what regulates their production and activity remains to be revealed.
3. Molecular and Hormonal Correlation
Sex hormones exert their effects on many cellular targets, including the immune system modulating directly and indirectly, the immune cell function, and the development and susceptibility of cells and tissues to autoimmune processes (Figure 1).
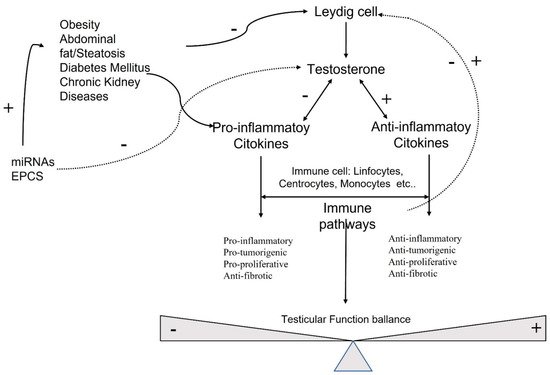
Figure 1. Molecular pathways involved in testicular function.
Testosterone is the most concentrated androgen in adult male serum, and the DHT–testosterone ratio is 1:10, but DHT is more potent than testosterone and cannot be converted to estrogens [66]. Androgens mediate their effects via binding to the androgen receptor (AR), a ligand-dependent transcription factor and a member of the nuclear receptor gene superfamily. Moreover, androgens can activate signaling pathways via non-DNA binding-dependent actions (Figure 2). The AR is expressed in many cells of the cardiovascular system, including endothelial cells and vascular smooth muscle [67]. Beyond its role in the development and expression of male phenotypes, the AR regulates immune function via modulating the transcription of several genes by DNA-binding-dependent and -independent mechanisms [68]. The AR is a signal transduction protein and transcription factor, and it is bound by heat shock proteins and chaperones in the cytoplasm until being bound by its ligands. Due to the differences in binding affinities and dissociation constants, AR:DHT complexes remains bound to AREs for longer than AR:testosterone complexes, further adding to the increased potency of DHT. AR regulates immune function via modulating the transcription of several genes by DNA-binding-dependent and -independent mechanisms (Figure 2).
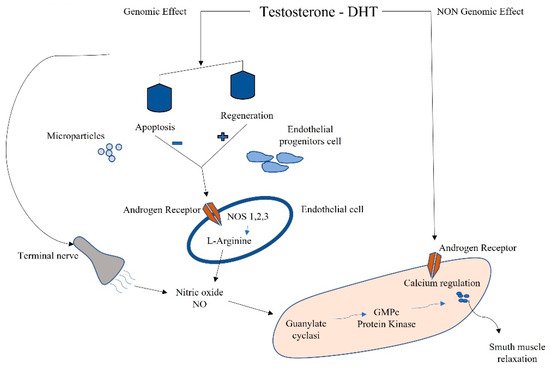
Figure 2. Schematic connection between immune microparticles, endothelial progenitor cells, and androgens in corpora cavernous endothelium.
Sex-hormone-driven, gender-specific modulation of EPCs is one of the mechanisms that accounts for cardiovascular risk. Higher levels of EPCs have been found in female genotypes due to estrogen modulation [69]. EPCs are also regulated by androgens; in fact, hypogonadal men have shown a reduction in circulating EPCs, which were found to be restored by pharmacological treatment with testosterone [70]. EPCs also have been negatively correlated to the testosterone/E2 ratio, and this complex interplay between estrogens and androgens in males influences apoptosis and regeneration. This negative correlation might even reveal a detrimental effect of testosterone on circulating EPCs (Figure 2) [8].
The testis has the two functions of spermatogenesis and steroidogenesis. Leydig, cells localized in the interstitium, as compact cell clusters closely associated with blood vessels, are necessary for testosterone production. Collagen fibers, fibroblasts, and mesenchymal cells constitute the interstitial tissue, which also contains the immune system (dendritic cells (DCs), T cells, B cells (rarely seen), and macrophages). Spermatogenesis occurs within the seminiferous tubules, where Sertoli cells target testosterone and play a pivotal role in germ cell proliferation and differentiation [8] (Figure 3).
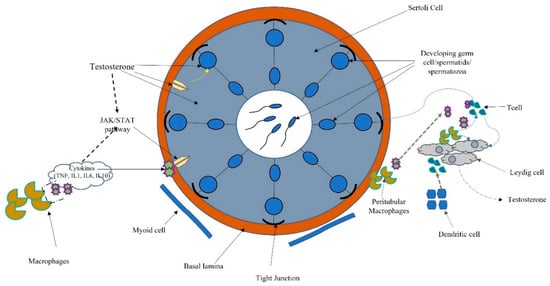
Figure 3. Schematic connection between immune system, cytokines, and hormonal pathways in testicular function and regulation.
Macrophages are the most prevalent cell type in the testicular interstitium and display a functional interaction with Leydig cells. Macrophages and DCs belong to the heterogeneous group of cells collectively called “antigen-presenting cells.” Antigen presentation plays a central role in initiating and maintaining appropriate immune response to antigens. Several molecular interactions between T cells and antigen-presenting cells ensure that T cells recognize antigenic peptides in a highly specific way. Activation of T cells results in the upregulation of cytokines and their receptors inducing activatory signals leading to cell proliferation and differentiation. T cells also regulate the expression of specific transcription factors associated with the development of many organ-specific autoimmune diseases and inflammatory tissue damage preventing pathogenic autoimmune responses [71] (Figure 3).
This entry is adapted from the peer-reviewed paper 10.3390/ijms222111908
This entry is offline, you can click here to edit this entry!
