Hepatocellular carcinoma (HCC) represents one of the main causes of cancer-related death worldwide. The transcription factor forkhead box O3 (FOXO3) has been related to hepatic diseases and tumor progression, but the exact role played by FOXO3 on HCC still remains unclear. Recently, a novel systematic review with meta-analysis revealed the potential diagnostic and prognostic value of FOXO3 in this primary liver cancer type.
- hepatocellular carcinoma
- liver cancer
- FOXO3
- diagnosis
- prognosis
- clinicopathological features
- hcc
- survival
- invasion
- forkhead box O3
1. Introduction
Liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of tumor-associated death[1]. About 85% of liver cancer cases correspond to hepatocellular carcinoma (HCC)[1][2], an aggressive tumor with high incidence and mortality[3][4][5]. Unfortunately, only a slight percentage of patients are eligible for curative treatments[2][3][4] and the prognosis of HCC remains very poor[6]. Therefore, finding new functional biomarkers could improve HCC patient outcomes.
The forkhead box O subgroup (FOXO) of transcription factors is composed of FOXO1, FOXO3, FOXO4 and FOXO6[7][8][9]. FOXO3 has shown to exert physiological and pathological functions by controlling the transcription of key target genes involved in multiple cellular processes[9][10][11]. Nonetheless, contradictory reports about the role of FOXO3 expression in cancer are found in literature[7][10]. In regard to HCC, certain articles sustain that abnormal FOXO3 overexpression could constitute an unfavorable hallmark[12][13][14][15][16][17]. Otherwise, other studies defend the association of low FOXO3 expression with poorer HCC patient outcomes[18][19][20].
With the aim of clarifying the role played by FOXO3 on HCC as well as of investigating the potential value of FOXO3 as a new biomarker in HCC, a recent systematic review with meta-analysis (PROSPERO registration code: CRD42021237321) evaluated the correlation of FOXO3 expression with HCC pathogenesis, survival parameters and clinicopathological factors. It needs to be mentioned that the study followed the PRISMA guidelines[21], determining the quality of the selected investigations with the Newcastle-Ottawa scale (NOS) criteria[22], and performing the statitiscal analysis using previously reported methodology[23][24].
2. Main Findings
♦ 2.1. FOXO3 and HCC development
Pooled results from the comparison of FOXO3 expression between tumor tissues and healthy normal liver samples proved that elevated levels of FOXO3 significantly correlate with HCC pathogenesis (OR, 15.98; 95% CI, 1.96–130.02; p = 0.01) (Figure 1a, Table 1).
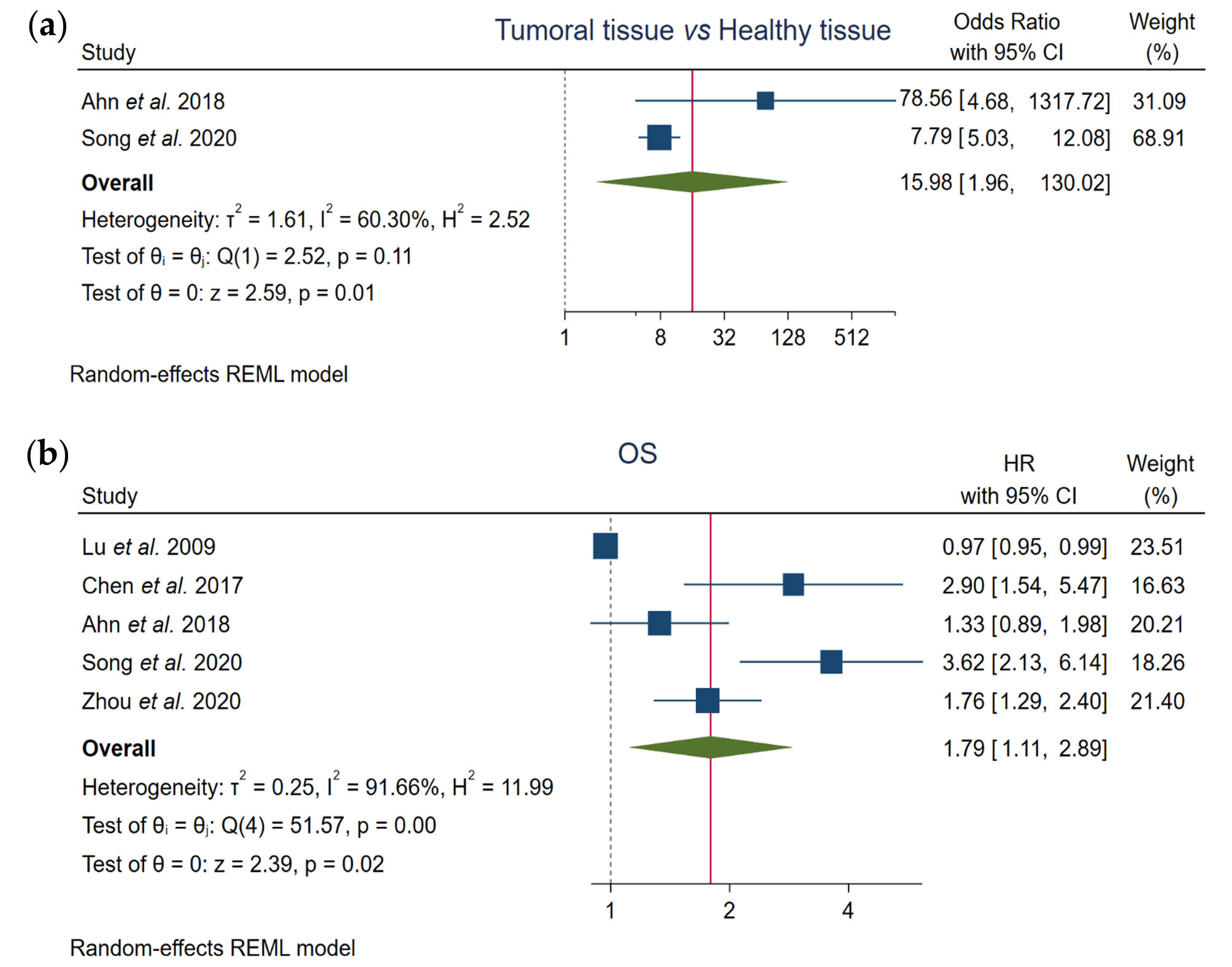
Figure 1. Assessment of the correlation of FOXO3 high expression with (a) tumor pathogenesis and (b) overall survival (OS) in HCC patients. CI, confidence interval; HR, hazard ratio; OS, overall survival; REML, Restricted Maximum Likelihood.
♦ 2.2. FOXO3 and OS
Based on the results of the total of the included articles, pooled data demonstrated that FOXO3 high levels significantly correlate with lower OS rates (HR, 1.79; 95% CI, 1.11–2.89; p = 0.02) (Figure 1b, Table 1).
♦ 2.3. FOXO3 and Clinicopathological Features
Initially, although all available data on clinicopathological factors were pooled and analyzed, the investigators did not observe any correlation of enhanced FOXO3 expression with different clinicopathological factors such as alpha-fetoprotein (AFP) levels, cirrhosis, gender, hepatitis B virus (HBV) infection, invasion, metastasis, tumor-node-metastasis (TNM) staging, tumor nodularity and tumor size (Figure 2, Table 1). However, certain parameters such as invasion showed an elevated heterogeneity among data. Therefore, subgroup analysis was subsequently conducted in order to investigate the heterogeneity causes and potentially find a significant correlation.
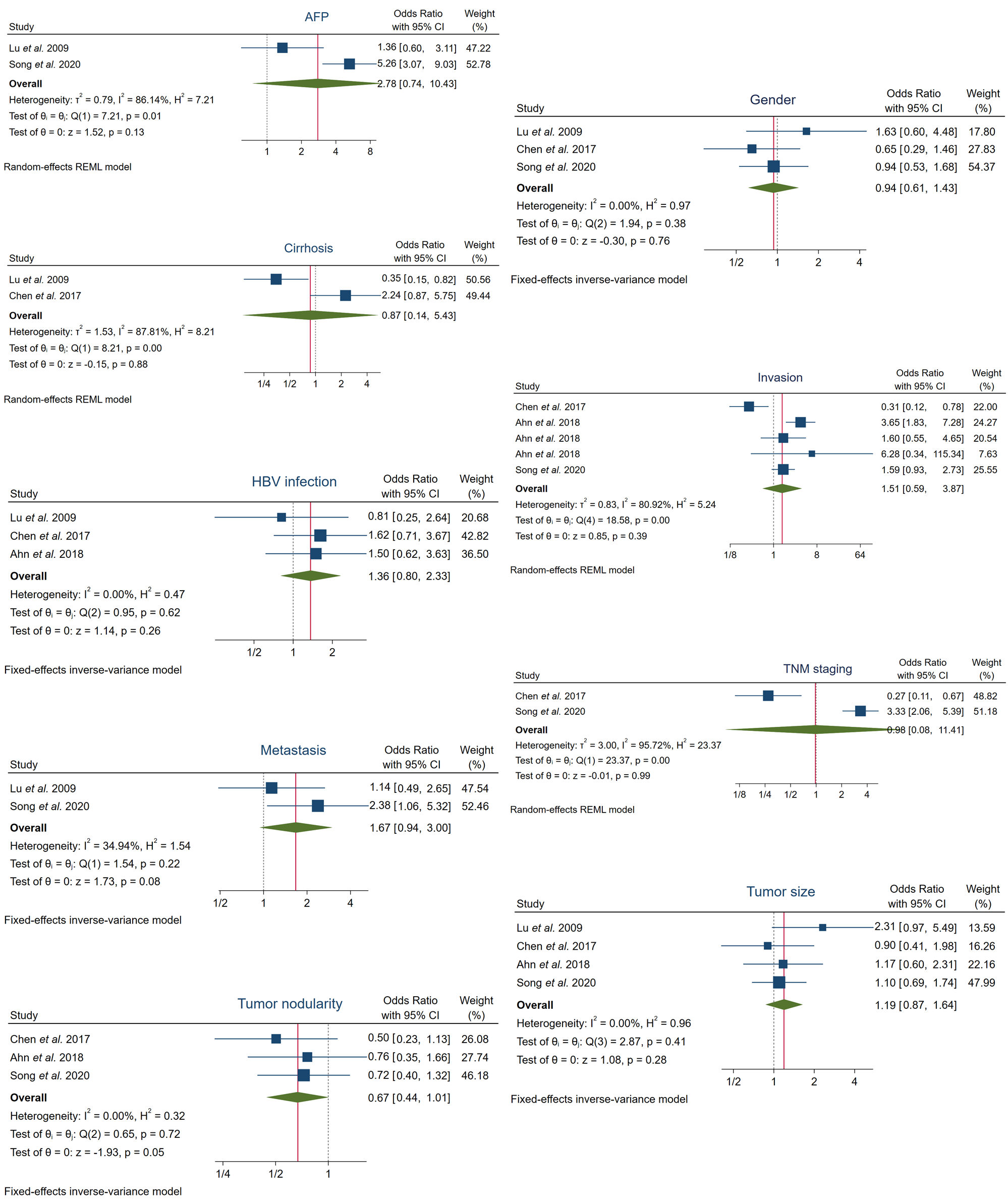
Figure 2. Analysis of the association between FOXO3 high expression and several clinicopathological features in HCC patients. AFP, alpha-fetoprotein; CI, confidence interval; HBV, hepatitis B virus; REML, Restricted Maximum Likelihood; TNM, tumor-node-metastasis.
Table 1. Evaluation of the association of enhanced FOXO3 levels with HCC pathogenesis, survival and clinicopathological features.
|
Parameter |
Number of Studies (n) |
Number of Cases (n) |
Samples with High FOXO3 Expression (n) |
High FOXO3 Expression (%) |
Pooled OR or HR |
Test for Heterogeneity |
Model Used |
|||
|
95% CI |
p-Value |
I2 |
Q-Test p-Value |
|||||||
|
HCC pathogenesis |
||||||||||
|
Tumoral tissue vs. Healthy tissue |
2 |
672 |
402 |
59.82% |
15.98 (1.96–130.02) |
0.01 |
60.30% |
0.11 |
REM |
|
|
OS |
5 |
1042 |
529 |
50.77% |
1.79 (1.11–2.89) |
0.02 |
91.66% |
0.00 |
REM |
|
|
Clinicopathological features |
||||||||||
|
AFP |
2 |
346 |
153 |
44.22% |
2.78 (0.74–10.43) |
0.13 |
86.14% |
0.01 |
REM |
|
|
Cirrhosis |
2 |
193 |
87 |
45.08% |
0.87 (0.14–5.43) |
0.88 |
87.81% |
0.00 |
REM |
|
|
Gender |
3 |
507 |
211 |
41.62% |
0.94 (0.61–1.43) |
0.76 |
0.00% |
0.38 |
FEM |
|
|
HBV infection |
3 |
378 |
207 |
54.76% |
1.36 (0.80–2.33) |
0.26 |
0.00% |
0.62 |
FEM |
|
|
Invasion |
5 |
890 |
497 |
55.84% |
1.51 (0.59–3.87) |
0.39 |
80.92% |
0.00 |
REM |
|
|
Metastasis |
2 |
400 |
168 |
42.00% |
1.67 (0.94–3.00) |
0.08 |
34.94% |
0.22 |
FEM |
|
|
TNM staging |
2 |
414 |
166 |
40.10% |
0.98 (0.08–11.41) |
0.99 |
95.72% |
0.00 |
REM |
|
|
Tumor nodularity |
3 |
603 |
287 |
47.60% |
0.67 (0.44–1.01) |
0.054 |
0.00% |
0.72 |
FEM |
|
|
Tumor size |
4 |
687 |
318 |
46.29% |
1.19 (0.87–1.64) |
0.28 |
0.00% |
0.41 |
FEM |
|
AFP, alpha-fetoprotein; CI, confidence interval; FEM, fixed-effects model; FOXO3, forkhead box O3; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HR, hazard ratio; OR, odds ratio; OS, overall survival; REM, random-effects model; TNM, tumor-node-metastasis.
♦ 2.4. Subgroup Analysis for OS and Invasion
In order to find the heterogeneity sources for every heterogeneous variable analyzed, subgroup analysis was carried out for parameters involving more than two original studies according to sample size, NOS score, patients’ origin or follow-up time.
Regarding OS parameter, heterogeneity was successfully resolved when NOS ≤ 6 or follow-up > 60 months (I2 = 15.43% and Q-test p = 0.28), also finding a significant correlation between high FOXO3 levels and poor OS (Figure 3a, Table 2). Moreover, heterogeneity in invasion was solved in the subgroup NOS = 6 and Korean provenance (I2 = 0.00% and Q-test p = 0.39), observing also for the first time a significant correlation with high levels of FOXO3 (OR, 2.95; 95% CI, 1.67–5.21; p = 0.00). Additionally, the elimination of Chen et al.[19] also led to an assumable heterogeneity and a significant association between FOXO3 overexpression and high probability of invasion (Figure 3b, Table 2).
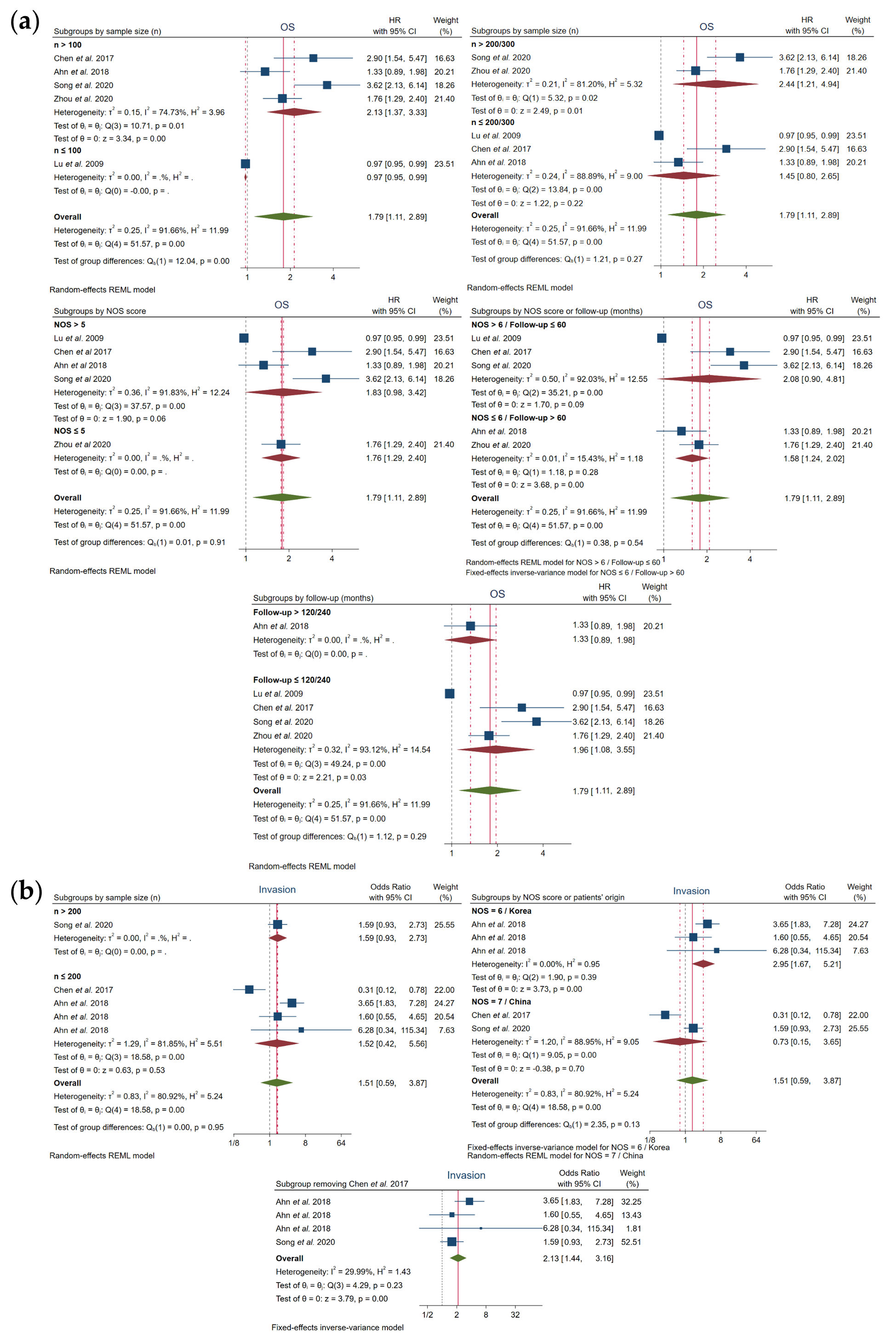
Figure 3. Subgroup analysis for (a) OS and (b) invasion. CI, confidence interval; HR, hazard ratio; NOS, Newcastle–Ottawa scale; OS, overall survival; REML, Restricted Maximum Likelihood.
Table 2. Subgroup analysis.
|
Subgroups |
Number of Studies (n) |
Number of Cases (n) |
Samples with High FOXO3 Expression (n) |
High FOXO3 Expression (%) |
Pooled OR or HR |
Test for Heterogeneity |
Model Used |
||
|
95% CI |
p-Value |
I2 |
Q-Test p-Value |
||||||
|
OS |
|||||||||
|
Sample size (n) |
|||||||||
|
n > 100 |
4 |
968 |
492 |
50.83% |
2.13 (1.37–3.33) |
0.00 |
74.73% |
0.01 |
REM |
|
n ≤ 100 |
1 |
74 |
37 |
50.00% |
0.97 (0.95–0.99) |
- |
- |
- |
- |
|
n > 200/300 |
2 |
679 |
329 |
48.45% |
2.44 (1.21–4.94) |
0.01 |
81.20% |
0.02 |
REM |
|
n ≤ 200/300 |
3 |
363 |
200 |
55.10% |
1.45 (0.80–2.65) |
0.22 |
88.89% |
0.00 |
REM |
|
NOS score (threshold 5) |
|||||||||
|
NOS > 5 |
4 |
677 |
438 |
64.70% |
1.83 (0.98–3.42) |
0.06 |
91.83% |
0.00 |
REM |
|
NOS ≤ 5 |
1 |
365 |
91 |
24.93% |
1.76 (1.29–2.40) |
- |
- |
- |
- |
|
NOS score (threshold 6) |
|||||||||
|
NOS > 6 |
3 |
490 |
317 |
64.69% |
2.08 (0.90–4.81) |
0.09 |
92.03% |
0.00 |
REM |
|
NOS ≤ 6 |
2 |
552 |
212 |
38.41% |
1.58 (1.24–2.02) |
0.00 |
15.43% |
0.28 |
FEM |
|
Follow-up (months) |
|||||||||
|
>60 |
2 |
552 |
212 |
38.41% |
1.58 (1.24–2.02) |
0.00 |
15.43% |
0.28 |
FEM |
|
≤60 |
3 |
490 |
317 |
64.69% |
2.08 (0.90–4.81) |
0.09 |
92.03% |
0.00 |
REM |
|
>120/240 |
1 |
187 |
121 |
64.71% |
1.33 (0.89–1.98) |
- |
- |
- |
- |
|
≤120/240 |
4 |
855 |
408 |
47.72% |
1.96 (1.08–3.55) |
0.03 |
93.12% |
0.00 |
REM |
|
Invasion |
|||||||||
|
Sample size (n) |
|||||||||
|
n > 200 |
1 |
227 |
92 |
40.53% |
1.59 (0.93–2.73) |
- |
- |
- |
- |
|
n ≤ 200 |
4 |
663 |
405 |
61.09% |
1.52 (0.42–5.56) |
0.53 |
81.85% |
0.00 |
REM |
|
NOS score |
|||||||||
|
NOS = 6 |
3 |
561 |
363 |
64.71% |
2.95 (1.67–5.21) |
0.00 |
0.00% |
0.39 |
FEM |
|
NOS = 7 |
2 |
329 |
134 |
40.73% |
0.73 (0.15–3.65) |
0.70 |
88.95% |
0.00 |
REM |
|
Patients’ origin |
|||||||||
|
China |
2 |
329 |
134 |
40.73% |
0.73 (0.15–3.65) |
0.70 |
88.95% |
0.00 |
REM |
|
Korea |
3 |
561 |
363 |
64.71% |
2.95 (1.67–5.21) |
0.00 |
0.00% |
0.39 |
FEM |
|
Without Chen et al.[19] |
|||||||||
|
|
4 |
788 |
455 |
57.74% |
2.13 (1.44–3.16) |
0.00 |
29.99% |
0.23 |
FEM |
CI, confidence interval; FEM, fixed-effects model; FOXO3, forkhead box O3; HR, hazard ratio; NOS, Newcastle–Ottawa scale; OR, odds ratio; OS, overall survival; REM, random-effects model.
3. Discussion
Asymptomatic presentation at early stages, deficient diagnostic techniques and post-therapy recurrence are common features of HCC, a lethal primary liver tumor with disappointing prognosis[2][6][15][16][19][25]. Even though increasing efforts are being put into biomarker discovery[24][25][26][27], effective molecules able to improve HCC detection and predict therapy response are still lacking. Meanwhile, it has been suggested that FOXO3 deregulation could be involved in cancer emergence[10][12][16] and progression[12][16][19], but the exact linkage between FOXO3 expression and primary liver cancer has not been clarified yet. Therefore, the current study was carried out to accurately determine the relationship of FOXO3 high expression with tumor development, survival rate and clinicopathological features, examining the potential usefulness of this factor as a diagnostic and prognostic biomarker for HCC monitoring.
This systematic review with meta-analysis, mainly accomplished with Chinese population, which is not surprising since most new HCC cases usually come from China[28], detected a significant correlation between FOXO3 high expression and HCC pathogenesis. Interestingly, Lu et al.[29] evidenced that enhanced FOXO3 expression and activity is associated with strong liver damage and overexpression of HCC-related genes. Additional reports from pre-clinical studies also indicated that FOXO3 upregulation is related to HCC oncogenicity[17][30][13]. Contrariwise, Wu et al.[31] described that reduction in FOXO3 nuclear translocation and activity could be involved in sepiapterin reductase-mediated HCC progression. Thus, this meta-analysis supports the findings reported by the majority of studies and suggests that upregulation of FOXO3 may constitute a suitable diagnostic factor able to complement classic techniques.
Furthermore, a significant association between FOXO3 overexpression and poor survival outcomes was registered in HCC patients, indicating that FOXO3 could constitute a negative prognostic factor in this tumor. Similar reports were shown in invasive ductal breast carcinoma[32], glioblastoma[33] and triple-negative breast cancer (TNBC) samples[34]. Otherwise, Zhao et al.[35] observed that FOXO3 downregulation could be linked with the enhancement of cell proliferation promoted by thyroid hormone receptor-interacting protein 6 (TRIP6). However, correlation between TRIP6 and FOXO3 expression in HCC individuals and its impact on survival rate were not assessed[35].
Additionally, it has been found that high FOXO3 levels may trigger HCC invasiveness. Different articles also determined that FOXO3 expression accentuates invasiveness and tumor expansion in glioblastoma[33], pancreatic cancer[36] and HeLa and melanoma MDA-MB-435 cells[37], being also correlated to perineural invasion in TNBC samples[34]. Contrariwise, FOXO3 oppositely impacted the invasive capabilities of breast tumors depending on the estrogen receptor α (ERα) status[38]. Moreover, Yang et al.[39] demonstrated the potential of bortezomib to inhibit cell migration and invasion by upregulating FOXO3 in cholangiocarcinoma and HCC models. However, these results were not tested in HCC patients, and bortezomib does not represent a major chemotherapeutic drug within the HCC field.
On the other hand, although no correlation was found between FOXO3 levels and any other evaluated clinicopathological feature, Chen et al.[40] indicated that FOXO3 could participate in the HBV-mediated HCC tumorigenesis. With regard to studies accomplished in other tumors, an investigation conducted with nasopharyngeal carcinoma samples observed that low FOXO3 expression correlates with advanced clinical stages and higher T stages, as well as with lymph node metastasis and distant metastasis[41]. Reduced FOXO3 levels in colorectal cancer[42], esophageal squamous cell carcinoma (ESCC)[43] and pancreatic ductal adenocarcinoma samples[44] have been also associated with more advanced disease. Deregulation of FOXO3 levels has shown to differentially influence lymph node metastasis in invasive ductal carcinoma[32], TNBC[34] and bladder carcinoma[45], finding that the interplay β-catenin-FOXO3 can be a metastasis promoter in colon cancer[46]. Besides, FOXO3 downregulation in ESCC patients accounted for lymph node metastasis[43], and its low expression correlated with a larger tumor size in gastric adenocarcinoma[47].
Altogether, these reports highlight the double-edged action played by FOXO3, finding that deregulation of FOXO3 expression and activity may lead to cancer promotion or suppression depending on the cancer type, cellular context or genomic profile.
4. Conclusions
In conclusion, this study proved for the first time that an enhanced FOXO3 expression could be an unfavorable clinical factor with diagnostic and prognostic significance in HCC, being associated with tumor development, poor OS and high risk of invasion. Therefore, the evaluation of FOXO3 levels could constitute a promising approach to optimize and complement HCC detection and, specifically, to guide patient surveillance and make an accurate prognosis.
Graphical Abstract. FOXO3, Forkhead box O3; HCC, hepatocellular carcinoma; OS, overall survival.
This entry is an adaptation from doi.org/10.3390/cancers13215349
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLO-BOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. https://doi.org/10.3322/caac.21660
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. https://doi.org/10.1038/s41572-020-00240-3
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2
- Kulik, L.; El-Serag, H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019, 156, 477–491. https://doi.org/10.1053/j.gastro.2018.08.065
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. https://doi.org/10.1002/ijc.32723
- Firkins, J.L.; Tarter, R.; Driessnack, M.; Hansen, L. A closer look at quality of life in the hepatocellular carcinoma literature. Qual. Life Res. 2021, 30, 1525–1535. https://doi.org/10.1007/s11136-021-02789-2
- Calissi, G.; Lam, E.W.-F.; Link, W. Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov. 2021, 20, 21–38. https://doi.org/10.1038/s41573-020-0088-2
- Coomans De Brachène, A.; Demoulin, J.-B. FOXO transcription factors in cancer development and therapy. Cell. Mol. Life Sci. 2016, 73, 1159–1172. https://doi.org/10.1007/s00018-015-2112-y
- Carbajo-Pescador, S.; Mauriz, J.L.; García-Palomo, A.; González-Gallego, J. FoxO proteins: Regulation and molecular targets in liver cancer. Curr. Med. Chem. 2014, 21, 1231–1246. https://doi.org/10.2174/0929867321666131228205703
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. https://doi.org/10.1186/s12943-018-0856-3
- Carbajo-Pescador, S.; Steinmetz, C.; Kashyap, A.; Lorenz, S.; Mauriz, J.L.; Heise, M.; Galle, P.R.; González-Gallego, J.; Strand, S. Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells. Br. J. Cancer 2013, 108, 442–449. https://doi.org/10.1038/bjc.2012.563
- Song, S.-S.; Ying, J.-F.; Zhang, Y.-N.; Pan, H.-Y.; He, X.-L.; Hu, Z.-M.; Wang, H.-J.; Dou, X.-B.; Mou, X.-Z. High expression of FOXO3 is associated with poor prognosis in patients with hepatocellular carcinoma. Oncol. Lett. 2020, 19, 3181–3188. https://doi.org/10.3892/ol.2020.11430
- Li, J.; Qin, X.; Wu, R.; Wan, L.; Zhang, L.; Liu, R. Circular RNA circFBXO11 modulates hepatocellular carcinoma progress and oxaliplatin resistance through miR-605/FOXO3/ABCB1 axis. J. Cell. Mol. Med. 2020, 24, 5152–5161. https://doi.org/10.1111/jcmm.15162
- Liang, C.; Dong, Z.; Cai, X.; Shen, J.; Xu, Y.; Zhang, M.; Li, H.; Yu, W.; Chen, W. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma. Cell Death Dis. 2020, 11, 1017. https://doi.org/10.1038/s41419-020-03233-y
- Zhou, Q.; Li, Z.; Song, L.; Mu, D.; Wang, J.; Tian, L.; Liao, Y. Whole-exome mutational landscape of metastasis in pa-tient-derived hepatocellular carcinoma cells. Genes Dis. 2020, 7, 380–391. https://doi.org/10.1016/j.gendis.2020.05.003
- Ahn, H.; Kim, H.; Abdul, R.; Kim, Y.; Sim, J.; Choi, D.; Paik, S.S.; Shin, S.-J.; Kim, D.-H.; Jang, K. Overexpression of forkhead box O3a and its association with aggressive phenotypes and poor prognosis in human hepatocellular carcinoma. Am. J. Clin. Pathol. 2018, 149, 117–127. https://doi.org/10.1093/ajcp/aqx132
- Liu, Z.; Li, Z.; Xu, B.; Yao, H.; Qi, S.; Tai, J. Long noncoding RNA PRR34-AS1 aggravates the progression of hepatocellular carcinoma by adsorbing microRNA-498 and thereby upregulating FOXO3. Cancer Manag. Res. 2020, 12, 10749–10762. https://doi.org/10.2147/CMAR.S263619
- Lin, Z.; Niu, Y.; Wan, A.; Chen, D.; Liang, H.; Chen, X.; Sun, L.; Zhan, S.; Chen, L.; Cheng, C.; et al. RNA m6A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020, 39, e103181. https://doi.org/10.15252/embj.2019103181
- Chen, Y.; Wang, J.; Zhang, L.; Yuan, P.; Lei, L.; Liu, D. Decreased expression of forkhead box O3 in human hepatocellular carcinoma and its prognostic significance. Int. J. Clin. Exp. Med. 2017, 10, 5851–5857
- Lu, M.; Ma, J.; Xue, W.; Cheng, C.; Wang, Y.; Zhao, Y.; Ke, Q.; Liu, H.; Liu, Y.; Li, P.; et al. The expression and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol. Oncol. Res. 2009, 15, 679–687. https://doi.org/10.1007/s12253-009-9171-z
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. https://doi.org/10.1136/bmj.n71
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 May 2021).
- Parmar, M.K.B.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. https://doi.org/10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
- Méndez-Blanco, C.; Fernández-Palanca, P.; Fondevila, F.; González-Gallego, J.; Mauriz, J.L. Prognostic and clinicopathological significance of hypoxia-inducible factors 1α and 2α in hepatocellular carcinoma: A systematic review with meta-analysis. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987071. https://doi.org/10.1177/1758835920987071
- Mansouri, V.; Razzaghi, M.; Nikzamir, A.; Ahmadzadeh, A.; Iranshahi, M.; Haghazali, M.; Hamdieh, M. Assessment of liver cancer biomarkers. Gastroenterol. Hepatol. Bed Bench 2020, 13, S29–S39.
- Sukowati, C.H.C.; Cabral, L.K.D.; Tiribelli, C.; Pascut, D. Circulating long and circular noncoding RNA as non-invasive diagnostic tools of hepatocellular carcinoma. Biomedicines 2021, 9, 90. https://doi.org/10.3390/biomedicines9010090
- Pratama, M.Y.; Visintin, A.; Crocè, L.S.; Tiribelli, C.; Pascut, D. Circulatory miRNA as a biomarker for therapy response and disease-free survival in hepatocellular carcinoma. Cancers 2020, 12, 2810. https://doi.org/10.3390/cancers12102810
- Gingold, J.A.; Zhu, D.; Lee, D.-F.; Kaseb, A.; Chen, J. Genomic profiling and metabolic homeostasis in primary liver cancers. Trends Mol. Med. 2018, 24, 395–411. https://doi.org/10.1016/j.molmed.2018.02.006
- Lu, M.; Hartmann, D.; Braren, R.; Gupta, A.; Wang, B.; Wang, Y.; Mogler, C.; Cheng, Z.; Wirth, T.; Friess, H.; et al. Oncogenic Akt-FOXO3 loop favors tumor-promoting modes and enhances oxidative damage-associated hepatocellular carcino-genesis. BMC Cancer 2019, 19, 887. https://doi.org/10.1186/s12885-019-6110-6
- Yang, L.; Deng, W.; Zhao, B.; Xu, Y.; Wang, X.; Fang, Y.; Xiao, H. FOXO3-induced lncRNA LOC554202 contributes to hepatocellular carcinoma progression via the miR-485-5p/BSG axis. Cancer Gene Ther. 2021. https://doi.org/10.1038/s41417-021-00312-w
- Wu, Y.; Du, H.; Zhan, M.; Wang, H.; Chen, P.; Du, D.; Liu, X.; Huang, X.; Ma, P.; Peng, D.; et al. Sepiapterin reductase promotes hepatocellular carcinoma progression via FoxO3a/Bim signaling in a nonenzymatic manner. Cell Death Dis. 2020, 11, 248. https://doi.org/10.1038/s41419-020-2471-7
- Chen, J.; Gomes, A.R.; Monteiro, L.J.; Wong, S.Y.; Wu, L.H.; Ng, T.T.; Karadedou, C.T.; Millour, J.; Ip, Y.-C.; Cheung, Y.N.; et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS ONE 2010, 5, e12293. https://doi.org/10.1371/journal.pone.0012293
- Qian, Z.; Ren, L.; Wu, D.; Yang, X.; Zhou, Z.; Nie, Q.; Jiang, G.; Xue, S.; Weng, W.; Qiu, Y.; et al. Overexpression of FoxO3a is associated with glioblastoma progression and predicts poor patient prognosis. Int. J. Cancer 2017, 140, 2792–2804. https://doi.org/10.1002/ijc.30690
- Rehman, A.; Kim, Y.; Kim, H.; Sim, J.; Ahn, H.; Chung, M.S.; Shin, S.-J.; Jang, K. FOXO3a expression is associated with lymph node metastasis and poor disease-free survival in triple-negative breast cancer. J. Clin. Pathol. 2018, 71, 806–813. https://doi.org/10.1136/jclinpath-2018-205052
- Zhao, W.; Dai, Y.; Dai, T.; Xie, T.; Su, X.; Li, J.; Zhou, X.; Meng, K.; Zhao, X. TRIP6 promotes cell proliferation in hepatocellular carcinoma via suppression of FOXO3a. Biochem. Biophys. Res. Commun. 2017, 494, 594–601. https://doi.org/10.1016/j.bbrc.2017.10.117
- Zhou, Y.; Chen, Y.; Ding, W.; Hua, Z.; Wang, L.; Zhu, Y.; Qian, H.; Dai, T. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. IUBMB Life 2018, 70, 276–290. https://doi.org/10.1002/iub.1699
- Storz, P.; Döppler, H.; Copland, J.A.; Simpson, K.J.; Toker, A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol. Cell. Biol. 2009, 29, 4906–4917. https://doi.org/10.1128/MCB.00077-09
- Sisci, D.; Maris, P.; Cesario, M.G.; Anselmo, W.; Coroniti, R.; Trombino, G.E.; Romeo, F.; Ferraro, A.; Lanzino, M.; Aquila, S.; et al. The estrogen receptor α is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. Cell Cycle 2013, 12, 3405–3420. https://doi.org/10.4161/cc.26421
- Yang, Z.; Liu, S.; Zhu, M.; Zhang, H.; Wang, J.; Xu, Q.; Lin, K.; Zhou, X.; Tao, M.; Li, C.; et al. PS341 inhibits hepatocellular and colorectal cancer cells through the FOXO3/CTNNB1 signaling pathway. Sci. Rep. 2016, 6, 22090. https://doi.org/10.1038/srep22090
- Chen, W.; Jiang, J.; Gong, L.; Shu, Z.; Xiang, D.; Zhang, X.; Bi, K.; Diao, H. Hepatitis B virus P protein initiates glycolytic bypass in HBV-related hepatocellular carcinoma via a FOXO3/miRNA-30b-5p/MINPP1 axis. J. Exp. Clin. Cancer Res. 2021, 40, 1. https://doi.org/10.1186/s13046-020-01803-8
- Shou, Z.; Lin, L.; Liang, J.; Li, J.-L.; Chen, H.-Y. Expression and prognosis of FOXO3a and HIF-1α in nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 585–593. https://doi.org/10.1007/s00432-011-1125-7
- Bullock, M.D.; Bruce, A.; Sreekumar, R.; Curtis, N.; Cheung, T.; Reading, I.; Primrose, J.N.; Ottensmeier, C.; Packham, G.K.; Thomas, G.; et al. FOXO3 expression during colorectal cancer progression: Biomarker potential reflects a tumour suppressor role. Br. J. Cancer 2013, 109, 387–394. https://doi.org/10.1038/bjc.2013.355
- Lu, Y.; Yu, J.; Yang, Z.; Zhu, G.; Gao, P.; Wang, H.; Chen, S.; Zhang, J.; Liu, M.; Niu, Y.; et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J. Exp. Clin. Cancer Res. 2018, 37, 301. https://doi.org/10.1186/s13046-018-0966-1
- Luo, X.; Yang, Z.; Liu, X.; Liu, Z.; Miao, X.; Li, D.; Zou, Q.; Yuan, Y. The clinicopathological significance of forkhead box P1 and forkhead box O3a in pancreatic ductal adenocarcinomas. Tumor Biol. 2017, 39, 1010428317699129. https://doi.org/10.1177/1010428317699129
- Wang, Y.; Kang, X.-L.; Zeng, F.-C.; Xu, C.-J.; Zhou, J.-Q.; Luo, D.-N. Correlations of Foxo3 and Foxo4 expressions with clinicopathological features and prognosis of bladder cancer. Pathol. Res. Pract. 2017, 213, 766–772. https://doi.org/10.1016/j.prp.2017.04.004
- Tenbaum, S.P.; Ordóñez-Morán, P.; Puig, I.; Chicote, I.; Arqués, O.; Landolfi, S.; Fernández, Y.; Herance, J.R.; Gispert, J.D.; Mendizabal, L.; et al. β-Catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med. 2012, 18, 892–901. https://doi.org/10.1038/nm.2772
- Yang, X.; Zhao, J.; Huang, C.; Wang, Q.; Pan, K.; Wang, D.; Pan, Q.; Jiang, S.; Lv, L.; Gao, X.; et al. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS ONE 2013, 8, e78158. https://doi.org/10.1371/journal.pone.0078158
