Arbuscular mycorrhizal (AM) fungi are biotrophic symbionts forming close relationships with an estimated 80% of terrestrial plants suitable as their host. Via an established AM fungal–host relationship, soil-bound nutrients are made available to the host plant through root cortical arbuscules as the site of exchange. At these sites, photosynthetic carbohydrates are provided to the AM fungus—carbohydrates that cannot be produced by the fungus. AM fungal–host symbiosis is very sensitive to soil disturbance, for example, agricultural tillage practices can damage and reduce AM fungal abilities to interact with a host and provide plant growth-promoting properties.
- arbuscular mycorrhizal fungi
- conservational
- glomalin
- soil quality
- sustainable
- symbiosis
- tillage
1. Introduction
2. Life Cycle
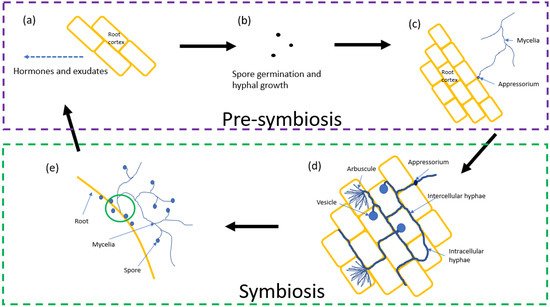
2.1. Pre-Symbiosis
2.2. Symbiosis
3. Mycorrhizal–Host Nutrient Exchange
3.1. Carbon
3.2. Nitrogen
3.3. Potassium
3.4. Phosphorus
3.5. Fatty Acids
3.6. Sulphur
3.7. Micronutrients
4. Arbuscular Mycorrhizal Fungi and Soil Structure
Soil Glomalin
5. Tillage
| Tillage Type | Equipment Employed | Tillage Characteristics | Impact on Soil and Arbuscular Mycorrhizal Fungi |
|---|---|---|---|
| Conventional | Mouldboard plough |
|
|
| Reduced | Rotary disc |
|
|
| Strip | Specialist equipment
|
|
|
| Zero | Direct seed drill |
|
|
5.1. Tillage and AM Fungi
5.2. Tillage, Glomalin and Soil Erosion
6. AM Fungi and Agrochemicals
| Method of Application | Product | Active Component | Crop Type | Effect on AM Fungal Abundance | Effect on Sporulation | Effect on Soil Glomalin Concentration |
|---|---|---|---|---|---|---|
| Seed treatment | Agrox™ | Captan | Pea (Pisum sativum), Chickpea (Cicer arietinum) | Neutral | No change | No change |
| Allegiance™ | Metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Apron Maxx RTA™ | Fludioxonil and metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Trilex AL™ | Trifloxystrobin and metalaxyl | Pea, Chickpea | Negative | No change | No change | |
| Vitaflo 280™ | Carbathiin and thiram | Pea, Chickpea | Negative | Inhibited | Reduced | |
| Crown™ | Carbathiin and thiabendazole | Pea, Chickpea | Negative | Inhibited | Reduced | |
| Thiram 75wp™ | Thiram | Pea, Chickpea | Neutral | No change | No change | |
| Plant application | Benomyl | 1-[(butyamino)carboyl-1H-benzimidazole-2yl] carbonate | Proso millet (Panicum miliaceum) | Negative | No change | No change |
| Bavistin | Methylbenzimidazol-2-yl carbonate | Proso millet | Negative | No change | No change | |
| Agrox™ | Captan | Proso millet | Positive | No change | No change | |
| Mancozeb | Manganese ethylenebis (dithiocarbomate) (polymatrix) complex zinc salt | Proso millet | Negative | No change | No change | |
| Soil drench | Benomyl | 1-[(butyamino)carboyl-1H-benzimidazole-2yl] carbonate | Cucumber (Cucumis sativus) | Negative | Inhibited | Reduced |
| Fenpropimorph | Rac-(2R,6S)-4-[(2E)-3-(-4-tert-butylphenyl)-2-methylpropyl]-2,6-dimethylmorpholine | Cucumber | Negative | Inhibited | Reduced | |
| Propiconazole | 1-((2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-20yl)methyl)-1H-1,2,4-triazole | Cucumber | Negative | Inhibited | Reduced | |
| Propiconazole and fenpropimorph | As above | Cucumber | Negative | Inhibited | Reduced |
Burrows and Ahmed [147] were able to show metalaxyl fungicides applied to maize (Zae mays L.) had varied influences on symbiotic AM fungal populations. Burrows and Ahmed [147] were able to show AM fungal–maize colonisation increased in the presence of metalaxyl alone and in combination with febuconazole. This is in contrast to the inhibitory effects of metalaxyl influences on AM fungal abundance and host colonisation of leeks (Allium ampeloprasum var. porrum), emphasising potential plant-derived AM fungal protection from metalaxyl.
Fungicide type and method of application have a profound effect on AM fungal abundance, sporulation, and host colonisation [148]. Table 2 presents several fungicides, their active component, along with their influence on AM fungi and their host crop. Soil drench applications of fungicides, direct application of fungicide to soils, were studied by Kjoller and Rosendahl [146] and demonstrated the reduced abundance of AM fungi in bulk soils and rhizosphere soils from ITS sequencing. Benomyl was used in both the studies by Kjoller and Rosendahl [146] and Channabasava and Jorquera [145]. However, Channabasava and Jorquera [145] applied benomyl fungicide to a developing crop and were able to show a reduction to AM fungal biomass but to a lesser degree than the soil drench application utilised by Kjoller and Rosendahl [146]. This is due to the quantity and duration of exposure of benomyl to soil-dwelling AM fungi. A further difference between the two studies, which will have a large implication towards fungicide application methods, is the difference in studied crop: cucumber (Cucumis sativus) and proso millet (Panicum miliaceum). Both of these crops are from different plant families and will interact with AM fungi in marginally different mechanisms. Therefore, the interaction and influences of fungicides towards AM fungi are not clear cut and are also dependant on the host crop.
Wang et al. [153] produced a study investigating phoxim pesticide with application concentrations ranging from 0 to 400 mg L−1 and applied directly to soils supporting the development of carrots (Daucus carota) and spring onions (Allium wakegi). Wang et al. [153] were able to show all phoxim applications reduced AM fungal colonisation of carrots but had no impact on AM fungal colonisation of spring onions. In a similar case to applications of fungicides, the host crop plays an invaluable role in the mitigation of agrochemical effects on the preservation of AM fungi communities. Furthermore, application time throughout the cropping year is also highly variable. Ipsilants et al. [150] applied a range of pesticides: azadirachtin, carbendazim, pyrethrum, spinosad, and terpens, across a range of days (20 to 90 days) post planting for a pepper (Piper nigrum) crop. Chemical applications at 20 days had the greatest degree of influence on AM fungi-mediated changes to root length. Spinosad and pyrethrum increased AM fungal colonisation and reduced root length by an additional 7% compared with control samples receiving no pesticide treatment. Such an increase in AM fungi colonisation may be the result of reduced community competition allowing Glomus mosseae, as identified by Ipsilants et al. [150], to increase host colonisation. All pesticide applications at day 90 produced increases in the degree of G. mosseae root colonisation, further suggesting reduced community competition within the rhizosphere has allowed G. mossaea to increase abundance and host interactions. Several studies have alluded to the reduced community of fungivorous nematodes from pesticide applications. Fungivorous nematodes were also studied to reduce the overall abundance of AM fungi. Pesticide-mediated reduction of fungivorous nematodes may be the causation for the increase in G. mossaea reported by Ipsilants et al. [150].
7. Abiotic Management of AM Fungi
| Plant Stressor | Crop | AM Fungal Inoculum | Crop Response |
|---|---|---|---|
| Salinity | Cucmis sativus L. | Glomus intraradices, Glomus mossaea | Increased chlorophyll content in leaves and overall biomass |
| Solanum lycopersicum L. | Glomus intraradices | Increased ion absorption and leaf chlorophyll | |
| Leymus chinensis | Glomus mosseae | Increased AM fungal colonisation, water content, and phosphorus and nitrogen uptake | |
| Triticum aestivum L. | Rhizophagus intraradices | Maintianed overall biomass, increased water uptake | |
| Heat | Triticum aestivum L. | Rhizophagus irregularis Rhizophagus intraradices |
Increased nutrient content and uptake, increase to overall biomass and water content |
| Zea mays L. | Rhizophagus intradices | Increased crop biomass and leaf chlorophyll | |
| Drought | Triticum aestivum L. | Glomus mosseae Glomus fasciculatum Rhizophagus irregularis Rhizophagus intraradices |
Increased crop biomass, ascorbic acid content, and leaf chlorophyll |
| Triticum aestivum | Glomus masseae | Increased crop biomass, ascorbic acid content, nitrogen and phosphorus metabolism, and leaf chlorophyll | |
| Triticum durum | Rhizophagus intraradices | Increased metal ions (copper, zinc, manganese) | |
| Zea mays | Rhizophagus intraradices | Increased absorption of phosphorus, potassium, nitrogen and magnesium |
Global climate change has driven an increased persistence in drought across many regions of the world. Drought drastically suppresses plant growth and reduces overall crop yields [169]. Many soil microbes, AM fungi, in particular, aid plant responses to drought. However, the interactions between plant and microbe in response to drought conditions, are not fully understood and are still an area requiring further investigation. Literature has demonstrated the increased water uptake from AM fungal branching mycelial networks through soil pores, not typically explored by host root systems, and the transport of water to the AM fungal host [159][170][171][172]. Duc et al. [170] and Auge et al. [158] reported the increased stomatal conducting leading to a higher transpiration rate, drawing up more AM fungal acquired water through the plant and mitigating drought conditions further for the host crop.
Studies and arable advice to reduce drought stress aim at increasing soil organic matter and moving soil management regimes towards conservational practices such as reducing till and ZT [155]. As shown by Wilkes et al. [173], the implementation of ZT practices conserves AM fungal populations and increases their influence on soil quality. Kozjek et al. [174] investigated the influence of drought on winter wheat via the construction of drought shelters over selected areas of developing crops and effectively reducing rainfall irrigation by 65%. Via molecular sequencing methodologies, Kozjek et al. [174] were able to show the predominate AM fungal genera, Acaulospora, Paraglomus and Funneliformis, associated with winter wheat both under drought shelters and in open field conditions. Furthermore, Kozjek et al. [174] demonstrated the adaptability of established AM fungal symbiosis under temporary drought between organic and CT farming systems. Reports of the adaptability of AM fungi in CT-managed soils are advantageous as CT-managed soils have the greatest degree of negative impacts and implications to AM fungal populations, leading to reductions in soil quality [173].
8. Summary
References
- Rillig, M.; Wright, S.; Eviner, V. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 2, 325–333.
- Kottke, I.; Nebel, M. The evolution of mycorrhiza-like associations in liverworts: An update. New Phytol. 2005, 167, 330–334.
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2020, 11, 1927.
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1996, 198, 97–107.
- Wright, S.F.; Frankee-Snyder, M.; Morton, J.B. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 1996, 181, 193–203.
- Pohanka, M.; Vlcek, V. Immunoassay of Glomalin by Quartz Crystal Microbalance Biosensor Containing Iron Oxide Nanoparticles. Int. J. Anal. Chem. 2020, 2020, 8844151.
- Lu, X.; Lu, X.; Lio, Y. Effect of Tillage Treatment on the Diversity of Soil Arbuscular Mycorrhizal Fungal and Soil Aggregate-Associated Carbon Content. Front. Microbiol. 2018, 9, 2986.
- Burri, K.; Groke, C.; Graf, F. Mycorrhizal fungi protect the soil form wind erosion: A wind tunnel study. Land Degrad. Dev. 2011, 24, 292–385.
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. Zero Tillage Systems Conserve Arbuscular Mycorrhizal Fungi, Enhancing Soil Glomalin and Water Stable Aggregates with Implications for Soil Stability. Soil Syst. 2021, 5, 4.
- Jiang, X.; Alan, L.; Wright, X.; Wang, F.; Liang, L. Tillage-induced changes in fungal and bacterial biomass associated with soil aggregates: A long-term field study in a subtropical rice soil in China. Appl. Soil Ecol. 2011, 48, 168–173.
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291.
- Zaller, J.G.; Heigl, F.; Ruess, L.; Grabmaier, A. Glyphosate herbicide affects belowground interactions between earthworms and symbiotic mycorrhizal fungi in a model ecosystem. Sci. Rep. 2014, 4, 5634.
- AHDB. Taking a Look at UK Crop Production 2020/21. 2020. Available online: https://ahdb.org.uk/news/taking-a-look-at-uk-crop-production-2020-21 (accessed on 15 July 2020).
- Berruit, A.; Lumini, E.; Baletini, B.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6.
- Wilkes, T.I.; Warner, D.J.; Davies, K.G.; Edmonds-Brown, V. Tillage, Glyphosate and Beneficial Arbuscular Mycorrhizal Fungi: Optimising Crop Management for Plant–Fungal Symbiosis. Agriculture 2020, 10, 520.
- Martins, S.; Medeiros, F.; Lakshamanan, V.; Bias, H. Impact of Seed Exudates on Growth and Biofilm Formation of Bacillus amyloliquefaciens ALB629 in Common Bean. Front. Microbiol. 2018, 8, 2631.
- Smith, S. Q&A: What are strigolactones and why are they important to plants and soil microbes? BMC Biol. 2014, 12, 19.
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 7.
- Buee, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe Interact. 2000, 13, 693–698.
- Thirkell, T.; Pastok, D.; Field, K. Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varieties according to cultivar and changes atmospheric carbon dioxide concentration. Glob. Chang. Biol. 2019, 26, 1725–1738.
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270.
- Balestrini, R.; Bonfante, P. Cell wall remodeling in mycorrhizal symbiosis: A way towards biotrophism. Front. Plant Sci. 2014, 5, 237.
- Ivanov, S.; Harrison, M.J. Accumulation of phosphoinositides in distinct regions of the periarbuscular membrane. New Phytol. 2019, 221, 2213–2227.
- Bonfante, P.; Anca, I.-A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 35, 363–383.
- Kariman, K.; Barker, S.J.; Tibbett, M. Structural plasticity in root-fungal symbioses: Diverse interactions lead to improved plant fitness. PeerJ 2018, 6, e6030.
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. A comparison of methodologies for the staining and quantification of intracellular components of arbuscular mycorrhizal fungi in the root cortex of two varieties of winter wheat. Access Microbiol. 2020, 1.
- Giovannetti, M.; Avio, L.; Fortuna, P.; Pellegrino, E.; Sbrana, C.; Strani, P. At the Root of the Wood Wide Web. Plant Signal. Behav. 2006, 1, 1–5.
- Lerat, S.; Lapointe, L.; Gutjahr, S.; Piche, Y.; Vierheilig, H. Carbon partitioning in a split-root system of arbuscular mycorrhizal plants is fungal and plant species dependent. New Phytol. 2003, 157, 589–595.
- Blanke, V.; Wagner, M.; Renker, C.; Lippert, H.; Michulitz, M.; Kuhn, A.J.; Buscot, F. Arbuscular mycorrhizas in phosphate-polluted soil: Interrelations between root colonization and nitrogen. Plant Soil 2011, 343, 379–392.
- Olsson, P.A.; Rahm, J.; Aliasgharzad, N. Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 2010, 72, 123–131.
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008.
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.W.; Brouillette, J.; Douds, D.D.; Lammers, P.J.; Shachar-Hill, Y. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003, 131, 1496–1507.
- Schubert, A.; Allara, P.; Morte, A. Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol. 2004, 1, 495–501.
- Doidy, J.; van Tuinen, D.; Lamotte, O.; Corneillat, M.; Alcaraz, G.; Wipf, D. The Medicago truncatula sucrose transporter family: Characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Mol. Plant 2012, 5, 1346–1358.
- Becard, G.; Doner, L.W.; Rolin, D.B.; Douds, D.D.; Pfeffer, P.E. Identification and quantification of trehalose in vesicular arbuscular mycorrhizal fungi by in vivo C-13 NMR and HPLC analyses. New Phytol. 1991, 118, 547–552.
- Morgan, J.B.; Connolly, E.L. Plant-Soil Interactions: Nutrient Uptake. Nat. Educ. Knowl. 2013, 4, 2.
- Johansen, A.; Jensen, E.S. Transfer of N and P from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biol. Biochem. 1996, 28, 73–81.
- Hodge, A.; Campbell, C.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299.
- Toussaint, J.-P.; St-Arnoud, M.; Charest, C. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can. J. Microbiol. 2004, 50, 251–260.
- Tian, J.; Dippold, M.; Pausch, J.; Blagodatskaya, E.; Fan, M.; Li, X.; Kuzyakov, Y. Microbial response to rhizodeposition depending on water regimes in paddy soils. Soil Biol. Biochem. 2013, 65, 195–203.
- Govindarajulu, M.; Pfeffer, P.; Jin, H.; Abubaker, J.; Douds, D.; Allen, J.; Bucking, H.; Lammers, P.; Schachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823.
- Calabrese, S.; Perez-Tienda, J.; Ellerbeck, M.; Arnould, C.; Chatagnier, O.; Boller, T.; Schussler, A.; Brachmann, A.; Wipf, D.; Ferrol, N. GintAMT3-a low-affinity ammonium transporter of the arbuscular mycorrhizal Rhizophagus irregularis. Front. Plant Sci. 2016, 7, 679.
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207.
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366.
- Garcia, K.; Zimmermann, S. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337.
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687.
- Olsson, P.A.; Hammer, E.C.; Wallander, H.; Pallon, J. Phosphorus availability influences elemental uptake in the mycorrhizal fungus Glomus intraradices, as revealed by particle-induced X-ray emission analysis. Appl. Environ. Microbiol. 2008, 74, 4144–4148.
- Casieri, L.; Lahmidi, N.A.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L. Biotrophic transportome in mutualistic plant fungal interactions. Mycorrhiza 2013, 23, 597–625.
- Rabie, G.G.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005, 4, 210–222.
- Estrada, B.; Aroca, R.; Maathuis, F.J.M.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782.
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440.
- Walder, F.; Boller, T.; Wiemken, A.; Courty, P.E. Regulation of plants’ phosphate uptake in common mycorrhizal networks: Role of intraradical fungal phosphate transporters. Plant Signal. Behav. 2016, 11, e1131372.
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725.
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26.
- Kobae, Y.; Ohmori, Y.; Saito, C.; Yano, K.; Ohtomo, R.; Fujiwara, T. Phosphate treatment strongly inhibits new arbuscule development but not the maintenance of arbuscule in mycorrhizal rice roots. Plant Physiol. 2016, 171, 566–579.
- Balzergue, C.; Chabaud, M.; Barker, D.G.; Becard, G.; Rochange, S.F. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front. Plant Sci. 2013, 4, 426.
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158.
- Wang, E.; Schornack, S.; Marsh, J.F.; Gobbato, E.; Schwessinger, B.; Eastmond, P.; Schultze, M.; Kamoun, S.; Oldroyd, G.E. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 2012, 22, 2242–2246.
- Feng, Z.; Liu, X.; Zhu, H.; Yao, Q. Responses of Arbuscular Mycorrhizal Symbiosis to Abiotic Stress: A Lipid-Centric Perspective. Front. Plant Sci. 2020, 11, 578919.
- Wewer, V.; Brands, M.; Dörmann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 2014, 79, 398–412.
- Feng, Z.; Liu, X.; Feng, G.; Zhu, H.; Yao, Q. Linking lipid transfer with reduced arbuscule formation in tomato roots colonized by arbuscular mycorrhizal fungus under low pH stress. Environ. Microbiol. 2020, 22, 1036–1051.
- Salvioli, A.; Ghignone, S.; Novero, M.; Navazio, L.; Venice, F.; Bagnaresi, P.; Bonfante, P. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J. 2016, 10, 130–144.
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175.
- Pons, S.; Fournier, S.; Chervin, C.; Becard, G.; Rochange, S.; Puech-Pages, V. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886.
- Ropars, J.; Toro, K.S.; Noel, J.; Pelin, A.; Charron, P.; Farinelli, L.; Marton, T.; Krüger, M.; Fuchs, J.; Brachmann, A.; et al. Evidence for the sexual origin of heterokaryosis in arbuscular mycorrhizal fungi. Nat. Microbiol. 2016, 1, 16033.
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.; Delaux, P.; Klingl, V.; Röpenack-Lahaye, E.; Wang, T.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 2017, 6, e29107.
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.L.S.; Driscoll, C.T.; Winkel, L.H. Reductions in the deposition of sulfur and selenium to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101.
- Gahan, J.; Schmalenberger, A. The role of bacteria and mycorrhiza in plant sulfur supply. Front. Plant Sci. 2014, 5, 723.
- Kertesz, M.; Mirleau, P. The role of microbes in plant sulphur supply. J. Exp. Bot. 2004, 55, 1939–1945.
- D’Hooghe, P.; Escamez, S.; Trouverie, J.; Avice, J.-C. Sulphur limitation provokes physiological and leaf proteome changes in oilseed rape that lead to perturbation of sulphur, carbon and oxidative metabolisms. BMC Plant Biol. 2013, 13, 23.
- Allen, J.; Shachar-Hill, Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2008, 149, 549–560.
- Buchner, P.; Takahashi, H.; Hawkesford, M. Plant sulphate transporters: Co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 2014, 55, 1765–1773.
- Cregut, M.; Piutti, S.; Slezack-Deschaumes, S.; Benizri, R. Compartmentalization and regulation of arylsulfatase activites in Streptomyces sp., Microbacterium sp. and Rhodococcis sp. soil isolates in response to inorganic sulfate limitation. Microbiol. Res. 2013, 168, 12–21.
- Joner, E.; Briomes, R.; Leyual, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234.
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265.
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12.
- Palmer, C.; Guerinot, M.L. A question of balance: Facing the challenges of Cu, Fe, and Zn homeostasis. Nat. Chem. Biol. 2009, 5, 333–340.
- Tamayo, E.; Gomez-Gallego, T.; Azcon-Aguilar, C.; Ferrol, N. Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front. Plant Sci. 2014, 4, 547.
- Lehman, A.; Rillig, M.C. Arbuscular mycorrhizal contributions to uptake of metal cations by cucumber plants at two levels of phosphorus supply. Plant Soil 2015, 278, 361–370.
- Schubler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; The Royal Botanic Garden Edinburgh: Edinburgh, Scotland; The Royal Botanic Garden Kew: Richmond, UK; Botanische Staatammlung Munich: München, Germany; Oregon State University: Corvallis, OR, USA, 2010.
- Gonzalez-Guerrero, M.; Escudero, V.; Saez, A.; Tejada-Jimenez, M. Transition metal transport in pland and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088.
- Watts-Williams, S.J.; Cavagnaro, T.R. Nutrient interactions and arbsuclar mycorrhizas: A meta-analysis of a mycorrhiza-defective mutant and wild-type tomato genotype pair. Plant Soil 2014, 384, 79–92.
- Watts-Williams, S.J.; Patti, A.F.; Cavagnaro, T.R. Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 2013, 371, 299–312.
- Jansa, J.; Mozafar, A.; Frossard, E. Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maise. Agronomie 2003, 23, 481–488.
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106.
- Li, A.-R.; Smith, S.E.; Smith, A.F.; Guan, K.-Y. Inoculation with arbuscular mycorrhizal fungi suppresses initiation of haustoria in the root hemiparasite Pedicularis tricolor. Ann. Bot. 2012, 109, 1075–1080.
- Larson, W.; Pierce, F. The dynamics of soil quality as a measure of sustainable management. In Defining Soil Quality for a Sustainable Environment; Wiley: Madison, WI, USA, 1994; pp. 37–51.
- Lovelock, C.E.; Wright, S.F.; Clark, D.A.; Ruess, R.W. Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape. J. Ecol. 2004, 92, 278–287.
- Bendini, S.; Pellegrino, E.; Avio, L.; Pellegrino, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1469–1494.
- Adeleke, A. Effect of Arbuscular mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria on Glomalin Production. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2010.
- Singh, P.; Singh, M.; Tripathi, B. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2012, 250, 663–669.
- Walley, F.; Gilliespie, A.; Adetona, A.; Germinda, J.; Farrell, R. Manipulation of rhizosphere organisms to enhance glomalin production and C sequestration: Pitfalls and promises. Can. J. Plant Sci. 2013, 94, 1025–1032.
- Prassad, M.; Chaudhary, M.; Ramakrishnan, S.; Mahawer, S. Glomalin: A miracle protein for soil sustainability. Indian Farmer 2018, 5, 1092–1100.
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H.; Eom, A.-H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 2013, 41, 121–125.
- Lombardo, L.; Palese, A.M.; Grasso, F.; Duffy, D.H., III; Bati, C.B.; Xiloyannis, C. Mechanical Tillage Diversely Affects Glomalin Content, Water Stable Aggregates and AM Fungal Community in the Soil Profiles of Two Differently Managed Olive Orchards. Biomolecules 2019, 9, 639.
- Sharifi, Z.; Azadi, N.; Rahimi, S.; Certini, G. The response of glomalin-related soil proteins to fire or tillage. Geoderma 2018, 329, 65–72.
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095.
- Sosa-Hernandez, M.; Leifheit, E.; Ingraffia, R.; Rillig, M. Subsoil Arbuscular Mycorrhizal Fungi for Sustainability and Climate-Smart Agriculture: A Solution Right under Our Feet? Front. Microbiol. 2019, 10, 744.
- Kabir, Z. Tillage or no-tillage: Impact on mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29.
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52.
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of Northeast China. PLoS ONE 2018, 13, e0199523.
- Zhao, X.; Liu, S.; Pu, C.; Zhang, X.; Xue, J.; Ren, Y.; Zhao, X.; Chen, F.; Lal, R.; Zhang, H. Crop yields under no-till farming in China: A meta-analysis. Eur. J. Agron. 2017, 84, 67–75.
- Ferrira, C.; da Silva Neta, C.; Pereira, M.; Guede, J.; Rosset, J.; Anjos, L. Dynamics of soil aggregation and organic carbon fractions over 23 years of no-till management. Soil Tillage Res. 2020, 198, 104533.
- Crittenden, S.J.; Poot, N.; Heinen, M.; van Balen, D.J.M.; Pulleman, M.M. Soil physical quality in contrasting tillage systems in organic and conventional farming. Soil Tillage Res. 2015, 154, 136–144.
- Sheehy, J.; Regina, K.; Alakukku, L.; Six, J. Impact of no-till and reduced tillage on aggregation and aggregate-associated carbon in Northern European agroecosystems. Soil Tillage Res. 2015, 150, 107–113.
- Dai, R.J.; Pang, X.G.; Zeng, X.D.; Wang, H.J. Soil carbon density and distribution and influencing factors in Shandong Province. Res. Environ. Sci. 2015, 28, 1449–1458.
- Ita, B.N.; Ariga, E.S.; Michieka, R.W.; Muiru, W.M. Comparative Efficiency of Tillage Practices in Maize. Curr. Agric. Res. J. 2014, 2.
- Moussa-Machraoui, S.B.; Errouissi, F.; Ben-Hammouda, M.; Nouira, S. Comparative effects of conventional and no-tillage management on some soil properties under Mediterranean semi-arid conditions in northwestern Tunisia. Soil Tillage Res. 2010, 106, 247–253.
- Moroke, T.S.; Dikinya, O.; Patrick, C. Comparative assessment of water infiltration of soils under different tillage systems in eastern Botswana. Phys. Chem. Earth 2009, 34, 316–323.
- Grange, I.; Prammanee, P.; Prasertsak, P. Comparative Analysis of Different Tillage Systems Used in Sugarcane (Thailand). Aust. Farm Bus. Manag. J. 2005, 2, 46–50.
- Stanila, S.; Drocas, I.; Molnar, A.; Ranta, O. Studies Regarding Comparative Fuel Consumption at Classical and Conservation Tillage. ProEnviron. Promediu 2013, 6, 199–202.
- Saglam, R.; Seven, L.; Kup, F. Comparative analysis of energy input-outputs of different tillage methods in second crop corn production. Not. Sci. Biol. 2020, 12, 356–365.
- Elliot, A.; Daniell, T.; Cameron, D.; Field, K. A commercial arbuscular mycorrhizal inoculm increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. New Phytol. Found. 2020, 3, 588–599.
- Brito, I.; Goss, M.J.; Carvalho, M.; Chatagnier, O.; Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Tillage Res. 2012, 121, 63–67.
- Kapoor, R. Arbuscular Mycorrhiza and Reactive Oxygen Species. In Arbuscular Mycorrhiza and Stress Tolerance of Plants; Neeraja, S., Ed.; Springer: Singapore, 2017; pp. 225–243.
- Douds, D.D.; Nagahashi, G. Signalling and Recognition Events Prior to Colonisation of Roots by Arbuscular Mycorrhizal Fungi. In Current Advances in Mycorrhizae Research; Podila, G.K., Douds, D.D., Eds.; APS Press: Saint Paul, MN, USA, 2000.
- Castillo, C.; Rubio, R.; Rouanet, J.L.; Borie, F. Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biol. Fertil. Soils 2006, 43, 83–92.
- Bernola, L.; Cange, C.; Way, M.; Gore, J.; Hardke, J.; Stout, M. Natural Colonization of Rice by Arbuscular Mycorrhizal Fungi in Different Production Areas. Rice Sci. 2018, 25, 169–174.
- Galvez, L.; Douds, D.; Wagoner, P. Tillage and farming system affect AM fungus populations, mycorrhizal formation, and nutrient uptake by winter wheat in a high-P soil. Am. J. Altern. Agric. 2001, 16, 152–160.
- Sharma-Poudyal, D.; Schlatter, D.; Yin, C.; Hulbert, S.; Paulitz, T. Long-term no-till: A major driver of fungal communities in dryland wheat cropping systems. PLoS ONE 2017, 12, e0184611.
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2008, 435, 824–827.
- Weetle, J.D.; Abril, M.; Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 2010, 5, e10899.
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evol. Int. J. Org. Evol. 2012, 66, 2961–2968.
- Wilson, G.W.T.; Rice, C.; Rillig, M.C.; Springer, A.; Hartnett, D. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461.
- Hontoria, C.; Velásquez, R.; Benito, M.; Almorox, J.; Moliner, A. Bradford-reactive soil proteins and aggregate stability under abandonedversus tilled olive groves in a semi-arid calcisol. Soil Biol. Biochem. 2009, 41, 1583–1585.
- Curaqueo, G.; Barea, J.; Acevedo, E.; Rubio, R.; Cornejo, P.; Borie, F. Effects of different tillage system on arbuscular mycorrhizal fungal propagules and physical properties in a Mediterranean agroecosystem in central Chile. Soil Tillage Res. 2011, 113, 11–18.
- Nautiyal, P.; Rajput, R.; Pandey, D.; Arunachalam, K.; Arunachalam, A. Role of glomalin in soil carbon storage and its variation across land uses in temperate Himalayan regime. Biocatal. Agric. Biotechnol. 2019, 21, 101311.
- Wright, S.F.; Green, V.S.; Cavigelli, M.A. Glomalin in aggregate size classes from three different farming systems. Soil Tillage Res. 2007, 94, 546–549.
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2016, 9, 685–692.
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessmentsand management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064.
- AHDB (Agriculture and Horticulture Development Board). Nutrient Management Guide (RB209), Section 4 Arable Crops; AHDB: Stoneleigh, UK, 2018.
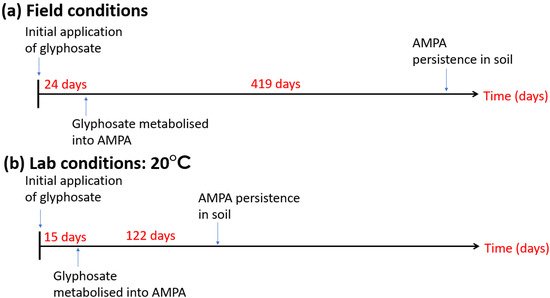
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, other herbicides, and transformation products in Midwestwern streams. J. Am. Water Resour. Assoc. 2005, 41, 323–332.
- Xu, J.J.; Fang, X.; Li, C.Y.; Yang, L.; Chen, X.Y. General and specialized tyrosine metabolism pathways in plants. aBIOTECH 2019, 1, 97–105.
- Palme, K.; Nagy, F. A new gene for auxin synthesis. Cell 2008, 133, 31–32.
- Neumann, E.; Kohls, S.; Landsberg, E.; Stock-Olivera, K.; Yamada, T.; Romheld, V. Relevance of glyphosate transfer to non-target plants via the rhizosphere. J. Plant Dis. Prot. 2006, 20, 963.
- Druille, M.; Omacini, M.; Golluscio, R.A. Glyphosate reduces spore viability and root colonization of arbuscular mycorrhizal fungi. Appl. Soil Ecol. 2013, 64, 99–103.
- Bott, S.; Tesfamariam, T.; Kania, A.; Eman, B.; Aslan, N.; Romheld, V.; Neumann, G. Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization. Plant Soil 2011, 342, 249–263.
- Druille, M.; Omacini, M.; Golluscio, R.A.; Cabello, M.N. Arbuscular mycorrhizal fungi are directly and indirectly affected by glyphosate application. Appl. Soil Ecol. 2013, 72, 143–149.
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120.
- Schonbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthasein atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380.
- Montgomery, H.J.; Monreal, C.; Young, J.C.; Seifert, K. Determination of soil fungal biomass from ergosterolanalyses. Soil Biol. Biochem. 2000, 32, 1207–1217.
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699.
- Marin, M.; Ybara, M.; Fe, A.; Garcia-Ferriz, L. Effects of arbsulr mycorrhizal fungi and pesticides on Cyara cardunculus growth. Agric. Food Sci. Finl. 2002, 11, 245–251.
- Jin, H.; Germida, J.; Walley, F. Suppressive effects of seed-applied fungicides on arbuscular mycorrhizal fungi (AMF) differ with fungicide mode of action and AMF species. Appl. Soil Ecol. 2013, 72, 22–30.
- Channabasava, H.; Jorquera, M. Effects of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L.). J. Soil Sci. Plant Nutr. 2015, 15, 35–45.
- Kjoller, R.; Rosendahl, S. Effects of fungicides on arbuscular mycorrhizal fungi: Differential responses in alkaline phosphatase activity of external and internal hyphae. Biol. Fertil. Soils 2000, 31, 361–365.
- Burrows, R.L.; Ahmed, I. Fungicide seed treatments minimally affect arbuscular-mycorrhizal fungal (AMF) colonization of selected vegetable crops. J. Biol. Sci. 2007, 7, 417–420.
- Rose, D.J.; Santra, D.K. Proso millet (Panicum miliaceum L.) fermentation for fuel ethanol production. Ind. Crops Prod. 2013, 43, 602–605.
- Thoeming, G.; Draeger, G.; Poehling, H.M. Soil application of azadirachtin and 3-tigloyl-azadirachtol to control western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) translocation and persistence in bean plants. Pest Manag. Sci. 2006, 62, 759–767.
- Ipsilants, I.; Samourelis, C.; Karpouzas, D. The impact of biological pesticides on arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2012, 45, 147–155.
- Rosendahl, S. Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 253–266.
- Gopal, M.; Gupta, A.; Arunachalam, V.; Magu, S.P. Impact of azadirachtin, an insecticidal allelochemical from neem on soil microflora, enzyme and respiratory activities. Bioresour. Technol. 2007, 98, 3154–3158.
- Wang, P.; Tong, R.; Shi, Z.; Xu, X.; He, X. Inoculations with arbuscular mycorrhizal fungi increase vegetable yields and decrease phoxim concentrations in carrot and green onion and their soils. PLoS ONE 2011, 6, e16949.
- Begum, N.; Qin, C.; Ahanger, M.; Raza, S.; Khan, M.; Ashraf, M.; Ahmed, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068.
- Evelin, H.; Devi, T.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470.
- Food and Agriculture Organization (FAO). Status of the Worlds’s Soil Resources (SWSR)—Main Report, United Nations; Food and Agriculture Organization: Rome, Italy, 2015.
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562.
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144.
- Liu, C.; Ravnskov, S.; Liu, F.; Rubæk, G.H.; Andersen, M.N. Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J. Agric. Sci. 2018, 156, 46–58.
- Bona, E.; Scarafoni, A.; Marsano, F.; Boatti, L.; Copetta, A.; Massa, N.; Gamalero, E.; D’Agostino, G.; Cesaro, P.; Cavaletto, M.; et al. Arbuscular mycorrhizal symbiosis affects the grain proteome of Zea mays: A field study. Sci. Rep. 2016, 6, 26439.
- Ingraffia, R.; Amato, G.; Sosa-Hernández, M.; Frenda, A.; Rillig, M.; Giambalvo, D. Nitrogen Type and Availability Drive Mycorrhizal Effects on Wheat Performance, Nitrogen Uptake and Recovery, and Production Sustainability. Front. Plant Sci. 2020, 11, 760.
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625.
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399.
- Abdi, N.; van Biljon, A.; Steyn, C.; Labuschagne, M.T. Bread Wheat (Triticum aestivum) Responses to Arbuscular Mycorrhizae Inoculation under Drought Stress Conditions. Plants 2021, 10, 1756.
- Hu, J.-L.; Lin, X.-G.; Wang, J.-H.; Shen, W.-S.; Wu, S.; Peng, S.-P.; Mao, T.-T. Arbuscular Mycorrhizal Fungal Inoculation Enhances Suppression of Cucumber Fusarium Wilt in Greenhouse Soils. Pedosphere 2010, 20, 586–593.
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981.
- García de León, D.; Vahter, T.; Zobel, M.; Koppel, M.; Edesi, L. Different wheat cultivars exhibit variable responses to inoculation with arbuscular mycorrhizal fungi from organic and conventional farms. PLoS ONE 2020, 15, e0233878.
- Reva, M.; Cano, C.; Herrera, M.; Bago, A. Arbuscular Mycorrhizal Inoculation Enhances Endurance to Severe Heat Stress in Three Horticultural Crops. HortScience 2021, 56, 396–406.
- Hasanuzzaman, M.; Gill, S.S.; Fujita, M. Physiological role of nitric oxide in plants grown under adverse environmental conditions. In Plant Acclimation to Environmental Stress; Tuteja, N., Gill, S.S., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; pp. 269–322.
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307.
- Chang, W.; Sui, X.; Fan, X.; Jia, T.; Song, F. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652.
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499.
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. The Tripartite Rhizobacteria-AM Fungal-Host Plant Relationship in Winter Wheat: Impact of Multi-Species Inoculation, Tillage Regime and Naturally Occurring Rhizobacteria Species. Plants 2021, 10, 1357.
- Kozjek, K.; Kundel, D.; Kushwaha, S.; Olsson, P.; Ahrén, D.; Fliessbach, A.; Birkhofer, K.; Hedlund, K. Long-term agricultural management impacts arbuscular mycorrhizal fungi more than short-term experimental drought. Appl. Soil Ecol. 2021, 168, 104140.
- Calvo-Polanco, M.; Sanchez-Romera, B.; Aroca, R.; Asins, M.; Declerk, S.; Dodd, I.; Martinez-Andujar, C.; Albacete, A.; Ruiz-Lozano, J. Exploring the use of recombinant inbred lines in conjunction with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57.
- Mitra, D.; Navendra, U.; Panneerselvam, U.; Ansuman, S.; Ganeshamurthy, A.N.; Divya, J. Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life Sci. Appl. Sci. 2019, 1, 1–10.
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686.
- Furze, J.; Martin, A.; Nasielski, J.; Thevathasan, N.; Gordon, A.; Isaac, M. Resistance and resilience of root fungal communities to water limitation in a temperate agroecosystem. Ecol. Evol. 2017, 7, 3443–3454.
- Camejo, D.; Rodriguez, P.; Morales, M.; Dell’Amico, J.; Torrecillas, A.; Alarcon, J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289.
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and auxin signaling pathways respond to high-temperate stress during another development as revealed by transcript profiling analysis in cotton. Plant Physiol. 2014, 164, 1293–1308.
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensiative maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerance cultivar. Front. Plant Sci. 2017, 8, 1056.
