Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anesthesiology
|
Health Care Sciences & Services
Plasma renin concentration as a marker of organ perfusion in several intensive care settings have shown a significant correlation between its increase and a lack of perfusion in critical tissues, especially in septic patients.
- plasma renin
- COVID-19
1. Case Presentation
During the first wave of COVID-19, we preliminarily enrolled a small cohort of subjects admitted to the Intensive Care Unit (ICU) with a diagnosis of COVID-19 and ARDS, without a previous hospitalization, from 1 April 2020 to 30 May 2020. All clinical data were gathered, and the plasma renin value was measured in the first 24 h (T0), in the following 72 h (T1), and after one week (T2). The percentage of survivors at the 90th day from admission was considered. We applied the ICU protocol with chloroquine, heparin prophylaxis, and tocilizumab at admission (if administrable). Data analysis was conducted using GraphPad Prism 8® (GraphPad Software 2365 Northside Dr. Suite 560 San Diego, CA 92108, USA). The difference in plasma levels over time was measured via a multiple-way ANOVA test, survival was measured at 90 days, and difficult mechanical weaning was defined as invasive mechanical ventilation for more than 15 days and/or a tracheotomy performed for respiratory reasons.
We enrolled eight critical COVID-19 patients. There were no significant clinical differences at admission (T0) among the eight patients enrolled between survivors and non-survivors regarding their Sequential Sepsis-related Organ Failure Assessment (SOFA) score and their Simplified Acute Physiology Score (SAPS II). Two of them were in therapy with ARB (angiotensin receptor blockers)/ACE inhibitor medications (Table 1). The mean level of renin plasma was lower in the survivor group and in the normal weaning group, as reported in Figure 1 with a p-value of 0.029*. Considering the low number of patients that the p value reported, only differences among groups are highlighted; however, the sample size does not allow clinical clear associations to be made.
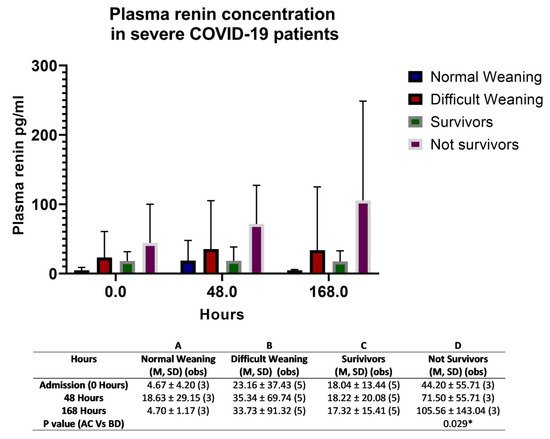
Figure 1. Plasma renin levels in COVID-19; the figure shows the difference between the survivors and non-survivors, and between normal weaning and difficult weaning. The results are expressed as the mean (M), standard deviation (SD), and number of observations (obs). The difference in plasma levels over time was measured via a multiple-way ANOVA test; p-value was considered significant if it was * < 0.05.
Table 1. Features of patients enrolled and plasma renin levels.
| Patient | Age (Year) |
SAPS II % (Percentage) |
Comorbidities | Plasma Renin pg/mL | SOFA SCORE (Points) | 90-Day Survivors | Difficult Weaning | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | ||||||
| Id 001 | 59 | 7.9% | Hypertension | 37.10 | 8.00 | 11.00 | 4 | 2 | 4 | Yes | Yes |
| Id 002 | 21 | 2.0 | Psychiatric, smoker | 9.50 | 52.30 | 6.00 | 4 | 3 | 2 | Yes | No |
| Id 003 | 63 | 7.9% | Prev. myocardial infarction (ACE receptors blocker) |
18.60 | 19.70 | 40.40 | 4 | 2 | 2 | Yes | Yes |
| Id 004 | 65 | 16.7% | Hypertension, hypothyroidism (ACE inhibitors) |
107.50 | 40.50 | 31.50 | 4 | 3 | 2 | No | Yes |
| Id 005 | 55 | 7.2% | None | 23.10 | 9.00 | 25.40 | 3 | 3 | 3 | Yes | Yes |
| Id 006 | 63 | 21.0% | None | 1.90 | 2.10 | 3.70 | 4 | 4 | 2 | Yes | No |
| Id 007 | 54 | 9.7% | Diabetes II, hypothyroidism | 2.60 | 1.50 | 4.40 | 3 | 4 | 3 | No | No |
| Id 008 | 61 | 9.7% | None | 22.5 | 172.50 | 206.70 | 3 | 4 | 3 | No | Yes |
2. Discussion
Several biomarkers have been proposed for evaluating the clinical severity of patients with COVID-19 with respiratory or neurological symptoms, such as interleukin 6, ferritin and LDH, or angiopoietin levels [1][2]. However, their measurement is often not simple or easy to perform, or their accuracy is not entirely known, and many conditions can alter their values [3]. Furthermore, RAAS dysregulation in COVID-19 patients has a dangerous role in cardiovascular complications [4]. In eight patients, we observed a higher plasma renin concentration in patients with difficulty weaning and in non-survivors; only one patient with a low value of plasma renin died, due to a sudden cerebral disease one day before ICU discharge. No difference in the production of renin was discovered in patients previously treated with sartan or ACE blockers (ARB or ACE inhibitors). Indeed, this is a preliminary observation, and it is not sufficient to demonstrate a possible biomarker role of plasma renin level in COVID-19 patients. However, it is interesting to observe how an increased plasma renin level during hospitalization usually follows difficult weaning as a possible expression of the severity of the disease. The variation of plasma renin levels and angiotensinogen in septic conditions is known, but settings such as COVID-19 infection have recently been investigated, showing a correlation with ACE2 receptor expression and functionality [5][6]. This cascade seems to correlate with the pulmonary severity of the disease. The downregulation of these receptors may increase angiotensin II stimulation, contributing to the deleterious hyperinflammatory reaction induced by the virus. This mechanism could be responsible for variations in the plasma renin concentration levels in this kind of patient. Furthermore, it is essential to underline the significant activation of the RAAS system in severe diseases as a physio-pathological phenomenon [7].
3. Conclusions
These are preliminary data, and it will be necessary analyze these associations with a larger cohort of patients. Observing the renin levels in each patient, the value of T0 and T2 could be marked as potential predictors of the evolution of the patients in terms of the need or not for mechanical ventilation, and whether they survive or not. Still, more patients would be needed to reach statistical significance. A possible early identification of the most critical subjects could be a valuable instrument for better allocation of resources and to improve quality of care. The value of plasma renin expression correlates with activation of the RASS system; in the near future, it will be interesting to have more data about its variation and value in COVID-19 patients.
This entry is adapted from the peer-reviewed paper 10.3390/biomed1020008
References
- Villa, E.; Critelli, R.; Lasagni, S.; Melegari, A.; Curatolo, A.; Celsa, C.; Romagnoli, D.; Melegari, G.; Pivetti, A.; Di Marco, L.; et al. Dynamic angiopoietin-2 assessment predicts survival and chronic course in hospitalized patients with COVID-19. Blood Adv. 2021, 5, 662–673.
- Melegari, G.; Rivi, V.; Zelent, G.; Nasillo, V.; De Santis, E.; Melegari, A.; Bevilacqua, C.; Zoli, M.; Meletti, S.; Barbieri, A. Mild to Severe Neurological Manifestations of COVID-19: Cases Reports. Int. J. Environ. Res. Public Health 2021, 18, 3673.
- Mesa, A.M.; César, E.C.; Martín-Montañez, E.; Alvarez, E.S.; Lopez, P.M.; Romero-Zerbo, Y.; Garcia-Fernandez, M.; Garrido, J.L.V. Acute Lung Injury Biomarkers in the Prediction of COVID-19 Severity: Total Thiol, Ferritin and Lactate Dehydrogenase. Antioxidants 2021, 10, 1221.
- Cooper, S.; Boyle, E.; Jefferson, S.; Heslop, C.; Mohan, P.; Mohanraj, G.; Sidow, H.; Tan, R.; Hill, S.; Woolard, J. Role of the Renin–Angiotensin–Aldosterone and Kinin–Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int. J. Mol. Sci. 2021, 22, 8255.
- Cure, E.; Ilcol, T.B.; Cure, M.C. Angiotensin II, III, and IV may be important in the progression of COVID-19. J. Renin-Angiotensin-Aldosterone Syst. 2020, 21, 147032032097201.
- Harky, A.; Chor, C.Y.T.; Nixon, H.; Jeilani, M. The controversy of using angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in COVID-19 patients. J. Renin-Angiotensin-Aldosterone Syst. 2021, 22, 1470320320987118.
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474.
This entry is offline, you can click here to edit this entry!
