Obesity is a worldwide disease characterized by an excessive body fat accumulation and by the presence of a subclinical chronic inflammatory status, which is associated with high morbidity and mortality rates. There are multiple pharmacological and non-pharmacological (exercise or dietary interventions) therapeutic strategies to face this disease. However, when these therapies failed, bariatric surgery is the most efficient treatment for obesity. In the last few years, different research studies have demonstrated a key role of gut microbiota, defined as all the microorganisms that habit in the digestive tract, in the development and progression of obesity. For that reason, going deepen in the knowledge of the link between bariatric surgery and gut microbiota could elucidate mechanistic and therapeutic approaches.
- bariatric surgery
- gut microbiota
- obesity
- metagenomics
- metabolomics
- short-chain fatty acids
INTRODUCTION
Obesity is one of the main public concerns worldwide, linked to increased rates of morbidity and mortality besides high resources demanding for public health systems [1]. Bariatric surgery is considered the gold standard treatment when non-surgical alternatives have failed, with a great performance to remedy the pathology in the short and in the long term [2][3].
The preponderant role of intestinal microbiota in the development of obesity is undoubtedly accepted in the current knowledge of the disease [4]. Under this perspective, the capacity of bariatric surgery to reshape gut microbiota as one of the mechanisms underlying its therapeutic success has been proposed and several findings reported in this sense. For example, a greater microbial gene richness and bacterial diversity, features associated to a healthy microbiota, have been observed after bariatric surgery [5]. Moreover, the causal role of bariatric surgery-mediated changes in gut microbiota composition in its anti-obesity effect has been demonstrated by the reduced fat mass gain in germ-free mice colonized with faecal microbiota from operated donors [6]. In fact, one of the main contributions of the gut microbiota to the instauration of obesity resides in energy extraction from the dietary nutrients. Weight loss by means of dietary interventions have demonstrated to modify gut microbiota, for example, counteracting the extensively reported increased Firmicutes/Bacteroidetes ratio in obesity and increasing beneficial Verrucomicrobia phylum [7], but also to promote functional changes in the microbiota and alter its derived metabolites [8][9]. Likewise, bariatric surgery could drive a shift of the metabolic capacity of the gut microbiota towards a lean-like phenotype both from a composition and from a functional point of view, related to the good outcomes of the procedure.
For the above-mentioned reasons, the main aim of this study is to evaluate the long-term effects of bariatric surgery in the faecal metagenome and metabolome of patients with severe obesity.
METHODS
To reach the proposed aim of this study, as it is shown in the figure below, faecal and blood samples were collected before and 4 years after bariatric surgery from patients with severe obesity and were processed for metabolomics and metagenomic analysis. Gut bacterial communities were identified by 16S rRNA Illumina MiSeq sequencing. Polar metabolites were detected and quantified by liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry were employed to determine the concentration of short chain fatty acids (SCFAs).
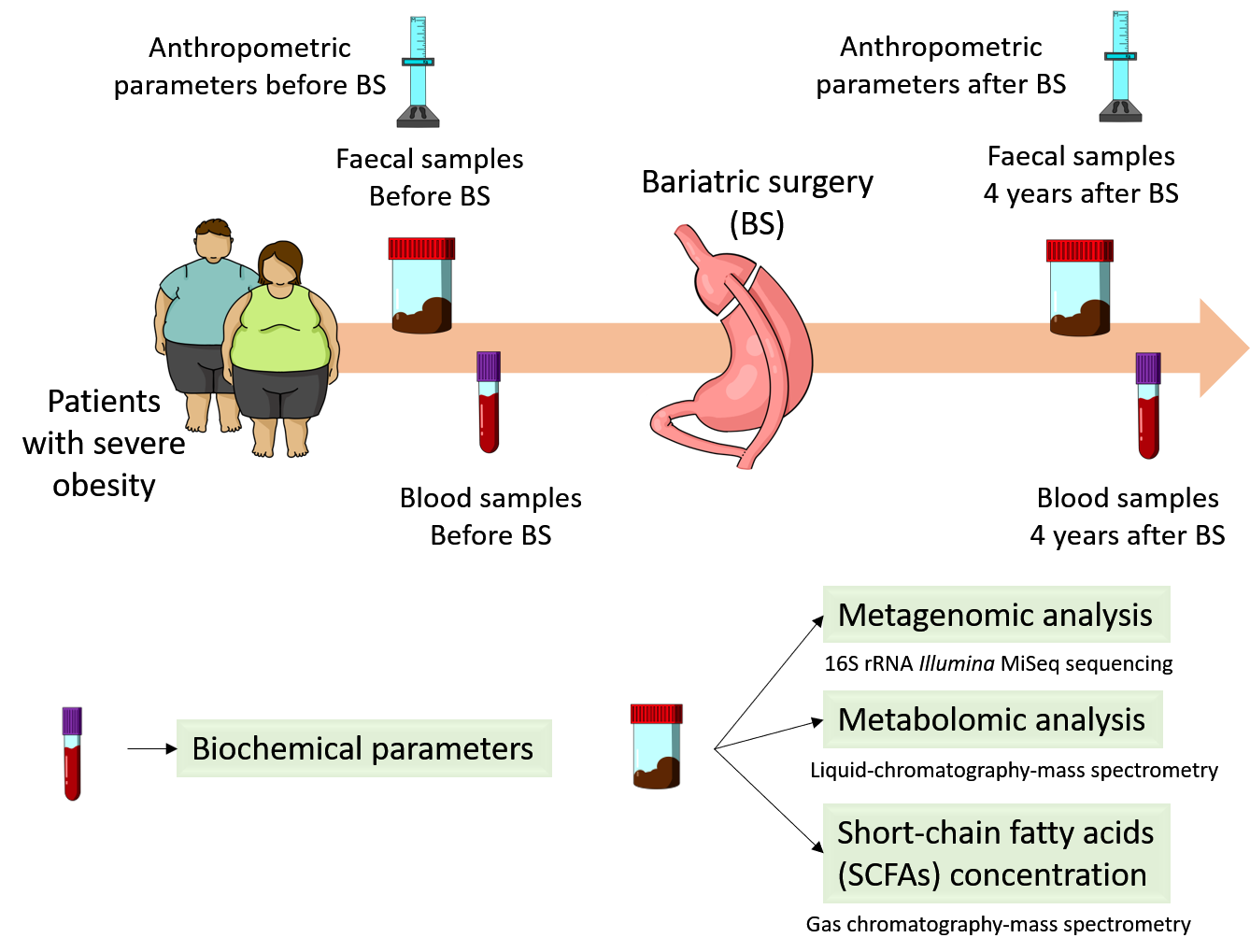 Figure 1. Experimental design.
Figure 1. Experimental design.
RESULTS AND DISCUSSION
Our results showed that bariatric surgery had a profound effect on biochemical and anthropometric parameters and modified the gut microbiota profile. The intervention increased Proteobacteria and Lenthispaerae phyla, whereas Firmicutes was reduced. Proteobacteria phylum has been associated with a beneficial profile characterized by decreased systemic inflammation and improved glucose homeostasis [5], and correlated with weight loss [10]. At family level, our results showed an increase in the abundance of Enterobacteriaceae, which has been negatively correlated with cholesterol levels in humans [11][12] as well as positively correlated with weight loss in animal models [13]. The abundance of the Lachnospiraceae family members has been positively correlated with BMI, suggesting that the decreased abundance of this family in our study may be related to weight loss [14].
Bariatric surgery also modified gut microbiota composition at the genus level. Butyricimonas, Parabacteroides and Slackia genera were increased and Coprococcus, Lachnospira, Lactococcus and Phascolarctobacterium showed an opposite pattern. High levels of Lactococcus have been associated with obesity and fasting plasma insulin [15]. Moreover, Acinetobacter overgrowth was present in patients under failed bariatric surgery [16], and in our study was positively correlated with LDL plasma concentration, so the significant reduction of this genus could indicate a more successful treatment. Parabacteroides has been negatively correlated with serum insulin concentration after bariatric surgery [17] and with BMI in our study, whereas high levels of Slackia have been detected in patients after Roux-en-Y gastric bypass [12]. Furthermore, increased abundance of Butyricimonas has been associated with less food addiction after bariatric surgery. Finally, Lachnospira decreased significantly in operated patients and was positively correlated with BMI, body fat and insulin levels.
The faecal metabolome of the patients with severe obesity was also modified due to bariatric surgery. Methyl acetoacetate, carbamoyl aspartate and serine phosphate increased, whereas metabolites such as 5-aminolevulinic acid, choline, citric acid, malic acid, taurine, TMAO and tropic acid decreased in faeces. Moreover, surgical intervention reduced the tricarboxylic acid cycle, glycine, serine and threonine metabolism, glyoxylate and dicarboxylate metabolism and tyrosine metabolism. These results suggest that branched-chain amino acids (BCAAs) and aromatic amino acids, as well as energetic metabolism, were downregulated with bariatric surgery, findings that have previously reported [18][19][20][21][22]. The reduction in BCAA levels as a consequence of bariatric surgery could be a normalization of the altered amino acid profile associated with obesity [18][19][20], which has been linked to an impairment of glucose homeostasis [18][23].
SCFAs are metabolites produced by gut microbiota fermentation from carbohydrates involved in various physiological process. High levels of these metabolites have been related to cardiometabolic and hepatic health [24][25][26], but also have been associated with gut dysbiosis, gut permeability and excess adiposity [27][28]. In our study, the SCFA faecal profiles of the patients after bariatric surgery were modified, with significant decreases of acetate, butyrate and propionate concentrations, in accordance with previous reports [29][30]. These SCFAs all positively correlated with BMI, reinforcing their reported role in obesity. Moreover, acetate, butyrate and propionate had a negative correlation with Butyricimonas and Parabacteroides genera, and a positive correlation with the Lachnospira genus.
Altogether, correlation analysis showed a metagenomic and metabolomic profile related to bariatric surgery that could be involved in the beneficial effects observed on biochemical and anthropometric parameters.
CONCLUSION
In conclusion, as it is summarized in the figure below, our findings point to bariatric surgery as a long-term modulator of gut microbiota, not only on its composition but also its functionality, promoting less efficient energy extraction from the diet as a possible mechanism linked to the persistent beneficia metabolic effects of a successful intervention.
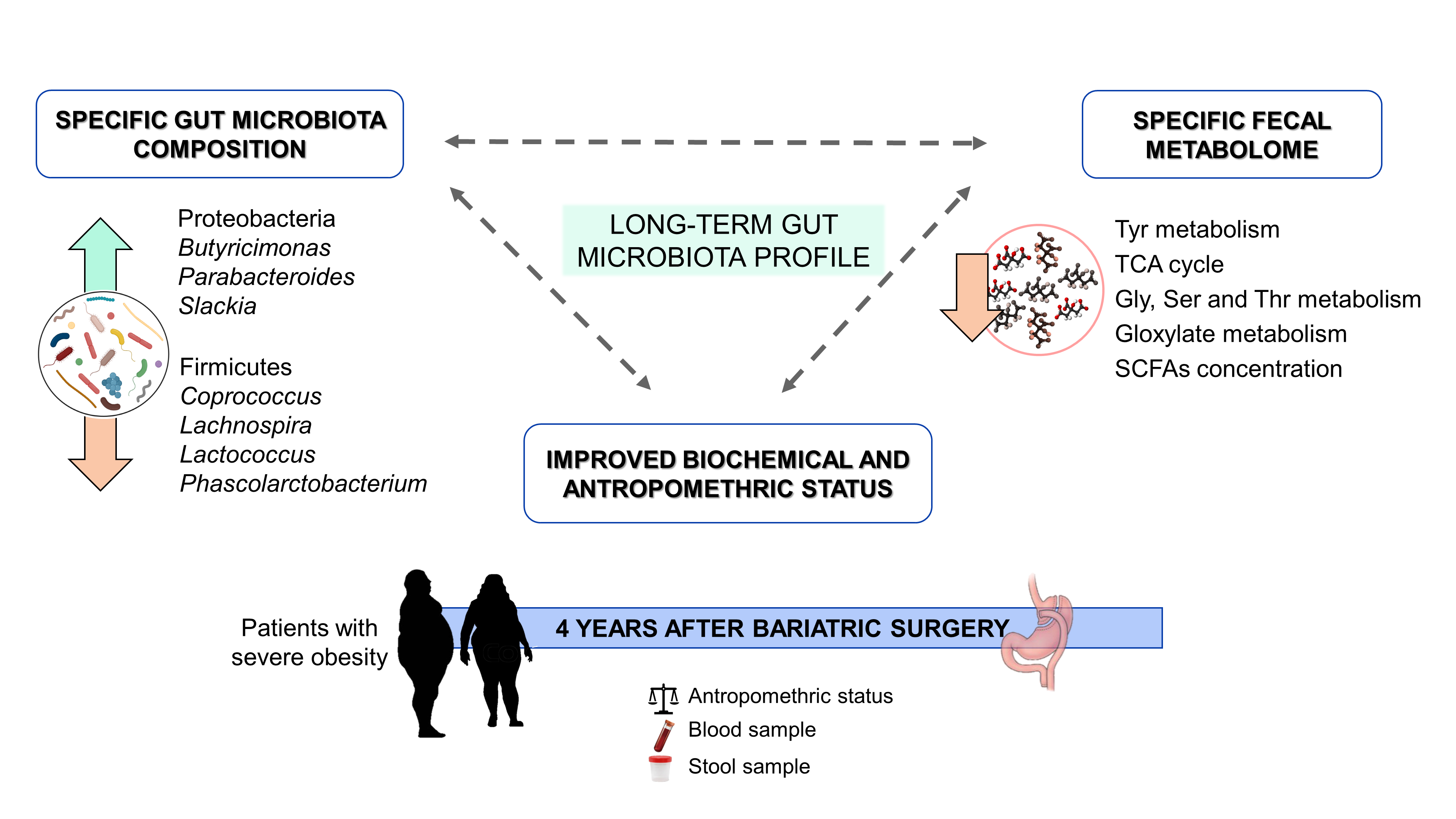 Figure 2. Graphical abstract.
Figure 2. Graphical abstract.
This entry is adapted from https://doi.org/10.3390/nu13082519
References
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615.
- Park, C.H.; Nam, S.J.; Choi, H.S.; Kim, K.O.; Kim, D.H.; Kim, J.W.; Sohn, W.; Yoon, J.H.; Jung, S.H.; Hyun, Y.S.; et al. Comparative efficacy of bariatric surgery in the treatment of morbid obesity and diabetes mellitus: A systematic review and network meta-analysis. Obes. Surg. 2019, 29, 2180–2190.
- Jiménez, A.; Ibarzabal, A.; Moizé, V.; Pané, A.; Andreu, A.; Molero, J.; de Hollanda, A.; Flores, L.; Ortega, E.; Lacy, A.; et al. Ten-year outcomes after Roux-en-Y gastric bypass and sleeve gastrectomy: An observational nonrandomized cohort study. Surg. Obes. Relat. Dis. 2019, 15, 382–388.
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, gut microbiota, and obesity: Links with host genetics and epigenetics and potential applications. Adv. Nutr. 2019, 10, S17–S30.
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut microbiota dysbiosis in human obesity: Impact of bariatric surgery. Curr. Obes. Rep. 2019, 8, 229–242.
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; Le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015, 22, 228–238.
- Rinninella, E.; Cintoni, M.; Raoul, P.; Ianiro, G.; Laterza, L.; Lopetuso, L.R.; Ponziani, F.R.; Gasbarrini, A.; Mele, M.C. Gut microbiota during dietary restrictions: New insights in non-communicable diseases. Microorganisms 2020, 8, 1140.
- Fabbiano, S.; Suárez-Zamorano, N.; Chevalier, C.; Lazarevi´c, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaïa, N.; Maresca, M.; et al. Functional gut microbiota remodeling contributes to the caloric restriction-induced metabolic improvements. Cell Metab. 2018, 28, 907–921.e7.
- Heianza, Y.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes in gut microbiota-related metabolites and longterm successful weight loss in response to weight-loss diets: The POUNDS lost trial. Diabetes Care 2018, 41, 413–419.
- Luijten, J.C.H.B.M.; Vugts, G.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. The importance of the microbiome in bariatric surgery: A systematic review. Obes. Surg. 2019, 29, 2338–2349.
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979.
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895.
- Li, J.V.; Ashrafian, H.; Bueter, M.; Kinross, J.; Sands, C.; Le Roux, C.W.; Bloom, S.R.; Darzi, A.; Athanasiou, T.; Marchesi, J.R.; et al. Metabolic surgery profoundly influences gut microbial—Host metabolic cross-talk. Gut 2011, 60, 1214–1223.
- Seganfredo, F.; Blume, C.; Moehlecke, M.; Giongo, A.; Casagrande, D.; Spolidoro, J.; Padoin, A.; Schaan, B.; Mottin, C.Weight-loss interventions and gut microbiota changes in overweight and obese patients: A systematic review. Obes. Rev. 2017, 18, 832–851.
- Qiao, Y.; Sun, J.; Xie, Z.; Shi, Y.; Le, G. Propensity to high-fat diet-induced obesity in mice is associated with the indigenous opportunistic bacteria on the interior of Peyer’s patches. J. Clin. Biochem. Nutr. 2014, 55, 120–128.
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martín-Núñez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952.
- Guo, Y.; Huang, Z.P.; Liu, C.Q.; Qi, L.; Sheng, Y.; Zou, D.J. Modulation of the gut microbiome: A systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 2018, 178, 43–56.
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322.
- Lopes, T.I.B.; Geloneze, B.; Pareja, J.C.; Calixto, A.R.; Ferreira, M.M.C.; Marsaioli, A.J. Omics prospective monitoring of bariatric surgery: Roux-en-Y gastric bypass outcomes using mixed-meal tolerance test and time-resolved 1H NMR-based metabolomics. Omi. A J. Integr. Biol. 2016, 20, 415–423.
- Lopes, T.I.B.; Geloneze, B.; Pareja, J.C.; Calixto, A.R.; Ferreira, M.M.C.; Marsaioli, A.J. Blood metabolome changes before and after bariatric surgery: A 1H NMR-based clinical investigation. Omi. A J. Integr. Biol. 2015, 19, 318–327.
- Palau-Rodriguez, M.; Tulipani, S.; Marco-Ramell, A.; Miñarro, A.; Jáuregui, O.; Sanchez-Pla, A.; Ramos-Molina, B.; Tinahones, F.J.; Andres-Lacueva, C. Metabotypes of response to bariatric surgery independent of the magnitude of weight loss. PLoS ONE 2018, 13, e0198214.
- Wijayatunga, N.N.; Sams, V.G.; Dawson, J.A.; Mancini, M.L.; Mancini, G.J.; Moustaid-Moussa, N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes. Metab. Res. Rev. 2018, 34, e3045.
- Narath, S.H.; Mautner, S.I.; Svehlikova, E.; Schultes, B.; Pieber, T.R.; Sinner, F.M.; Gander, E.; Libiseller, G.; Schimek, M.G.; Sourij, H.; et al. An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS ONE 2016, 11, 1–18.
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849.
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061.
- Carbajo-Pescador, S.; Porras, D.; Garcia-Mediavilla, M.V.; Martinez-Florez, S.; Juarez-Fernandez, M.; Cuevas, M.J.; Mauriz, J.L.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. DMM Dis. Model. Mech. 2019, 12, dmm039206.
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019, 11, 51.
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients 2019, 11, 2512.
- Patrone, V.; Vajana, E.; Minuti, A.; Callegari, M.L.; Federico, A.; Loguercio, C.; Dallio, M.; Tolone, S.; Docimo, L.; Morelli, L. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front. Microbiol. 2016, 7, 200.
- Liou, A.P.; Paziuk, M.; Luevano, J.M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41.
