In the field of synthesis and processing of noble metal nanoparticles, the study of the bottom-up method, called Ultrasonic Spray Pyrolysis (USP), is becoming increasingly important. This review analyses briefly the features of USP, to underline the physical, chemical and technological characteristics for producing nanoparticles and nanoparticle composites with Au and Ag. The main aim is to understand USP parameters, which are responsible for nanoparticle formation. There are two nanoparticle formation mechanisms in USP: Droplet-To-Particle (DTP) and Gas-To-Particle (GTP). This review shows how the USP process is able to produce Au, Ag/TiO2, Au/TiO2, Au/Fe2O3 and Ag/(Y0.95 Eu0.05)2O3 nanoparticles, and presents the mechanisms of formation for a particular type of nanoparticle. Namely, the presented Au and Ag nanoparticles are intended for use in nanomedicine, sensing applications, electrochemical devices, and catalysis, in order to benefit from their properties, which cannot be achieved with identical bulk materials. The development of new noble metal nanoparticles with USP is a constant goal in Nanotechnology, with the objective to obtain increasingly predictable final properties of nanoparticles.
- Ultrasonic Spray Pyrolysis
- Nanotechnology
- Noble Metals Nanoparticles
- Formation Mechanism
- Characterisation
- Properties
1. Introduction
The processing of aerosols (solid or liquid particles suspended in gas) and obtaining various types of fine particles and nanomaterials from aerosols has been known for a long time. These materials are meant for diverse uses in different fields, from nanomedicine, sensing applications, electrochemical devices, catalysis, energy conversion and storage, to name a few [1][2][3][4]. Generally, the basic principle of aerosol formation arises from a solution that contains dissolved material (usually salts of various metals)—which are commonly called precursors. In the synthesis, the powder product is atomised from an aerosol composed of liquid droplets, which are then transported with a carrier gas to the reactor where the chemical transformation occurs. The chemical composition of the precursor depends on the chemical composition of the fine powder we want to obtain and the type of processing method used, such as spray drying, Spray Pyrolysis, Flame Spray Pyrolysis, thermal decomposition, micronisation or gas atomisation [5][6][7]
Spray drying techniques are used widely in the food industry, such as for the production of milk or fruit powders and for flavour ingredients, as well as in pharmaceutics’ production [5][8]. This technique is a low-cost, environmentally friendly method with proven scalability, and is well known in Chemical Engineering [9].
Spray Pyrolysis, Flame Spray Pyrolysis and aerosol decomposition or thermolysis, generally involve atomisation of metal salt solutions as the precursor for the final metal or metallic oxide particles, and subjecting these liquid droplets to a high temperature in a furnace or a flame. Water or organic solutions with different metal salts may be used, from chlorides, acetates, nitrates, bromides, sulphides, hydroxides, etc., depending on the necessary composition and structure of the final particles [5][10].
Aerosol assisted sol–gel processes combine the sol–gel chemistry with aerosol processing [11]. In principle, each aqueous aerosol droplet may be used as a single, micron-sized sol–gel reactor, capable of producing different particle structures, such as mesoporous particles of various materials and chemical compounds [12][13][14]. In gas atomisation, liquid metal is sprayed through a nozzle and cooled rapidly to produce solid small spherical particles of the used metal or alloy [6][7].
Most of these techniques require the atomisation of a liquid solution into micron-sized droplets. The generation of such droplets is achievable by using pressure, electrostatic fields, centrifugal force or ultrasound [5].
This review is focused on Ultrasonic Spray Pyrolysis (USP), which combines the ultrasound used for dispersing the precursor solution into droplets and chemical decomposition of the dissolved material inside the droplets at elevated temperatures, resulting in the formation of fine powder. In the USP reactor the evaporation of solvent from droplets occurs first, in the next stage the remaining solvent inside the dry droplets is decomposed chemically via pyrolysis, and, in the final stage, fine particles are obtained through the sintering process. The advantage of the USP method is the simplicity of setting up individual process segments and changing their configuration, continuous nanomaterials’ synthesis, and the possibility of synthesising nanoparticles from various raw materials. The disadvantage is the low efficiency, due to losses of the dissolved material on the construction elements of the USP device.
Currently, there is a need for scaled-up production of nanoparticles, able to take up the increasing quantities of nanoparticles required for advanced applications. Technologies for generating nanoparticle powders and suspensions have existed for several decades [15], and are being improved upon continually, while novel approaches are also being studied [16][17]. One potentially reliable method for the large scale synthesis of nanoparticles is the USP process [18]. This process is considered to be relatively cost-effective, and easily scalable from the laboratory to an industrial level. Several different types of nanoparticles and structures can also be produced by USP, such as solid or hollow nanoparticles, core–shell and ball-in-ball structures, etc. [4][19][20][21][22], giving an additional advantage to this production method. Yolk–shell-structured [23][24], mesoporous [25] and multicomponent composite particles [26][27] were also proven to be synthesised with this method, by a group focusing on Li-ion and Na-ion battery applications of these particles. Depending on the particle composition, the formation of mesoporous or multicomponent structures can also be facilitated with subsequent processing of the initial particles produced by USP. The process is also used extensively for thin film deposition [21][28], with multiple layers [29]. The latest scientific finding reported [30] that a fully solution-based fabrication process exploiting USP and spin coating techniques, owing to their simplicity, high degree of freedom for mixing metal oxide precursor salts, and larger area deposition, opens the potential application of solution-processed transistors for low-cost electronic devices.
In our long-term investigation and experimental work, we have used USP to produce several metallic and metal oxide particles with micron and nano sizes, ranging from Au (from Au salts and from gold scrap), Ag, Ag/TiO2, Au/TiO2, ZnO, Fe/Au, Co, Cr and rare earth metals. For Au nanoparticles (AuNPs) we have also investigated extensively the cytotoxic and immunomodulatory properties of these particles for biomedical applications [31][32][33], as well as their behaviour when exposed to in situ heating during TEM observation [34], and their use in printing inks for Electronics (not yet published). We have also applied Au and ZnO nanoparticles in PMMA composites for advanced dental materials for removable prostheses [35]. The Ag/TiO2 and Au/TiO2 particles were produced for use as catalysts, and rare earth metals for photocatalytic applications [36][37].
2. Ultrasonic Spray Pyrolysis
The main elements of the conventional USP device are the ultrasonic generator or nebuliser, the reactor furnace, and a system for nanoparticle collection and cooling (Figure 1). Ultrasonic nebulisers are the most efficient amongst other types of nebulisers for nanomaterial production, such as pneumatic and electrostatic, while also being affordable and having a low droplet velocity. Pneumatic nebulisers produce droplets by expanding a pressurised liquid. These nebulisers have a high droplet output of large sizes around ≈50 µm, and the droplet sizes and size distribution are difficult to control [38]. Electrostatic nebulisers use an electrostatic field to extract droplets from a liquid [28]. They have a low productivity, and can only be used on conductive liquids. Ultrasonic nebulisers produce droplets in a narrow size distribution, less than 10 µm, with acceptable productivity. Ultrasonic spray nozzles generate more stable and uniform droplets, but cannot produce droplets as small as ultrasonic nebulisers [38]. As such, ultrasonic nebulisers have good characteristics regarding their droplet output, and are used commonly in Spray Pyrolysis processes (Table 1).
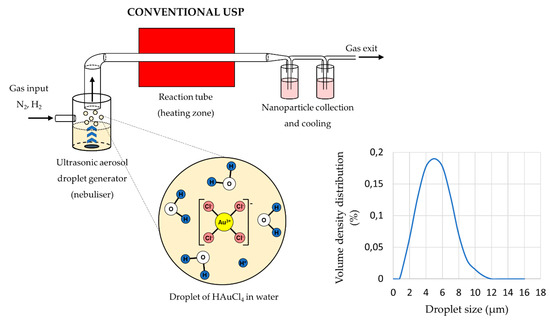
Figure 1. Aerosol droplet formation from a starting solution with ultrasound, an example of gold chloride precursor for AuNP synthesis (Assumption: The ion states inside the aerosol droplets are the same as in the starting solution, adapted from [39][40]).
Table 1. Comparison of production yields of selected aerosol routes for particle synthesis.
| Particle Production Method | Typical Particle Sizes | Possible Production Yield |
|---|---|---|
| Ultrasonic Spray Pyrolysis | Nanometer to micron-sized particles, 10 nm–5 µm | 0.1–100 kg/d [28][41] |
| Flame Spray Pyrolysis | Nanometer to submicron-sized particles, 5–500 nm | 1–25 kg/d, derived from [40] |
| Spray Pyrolysis—pneumatic nebuliser | Micron-sized particles or larger, >1 µm | >5 t/d [28] |
| Spray Pyrolysis—electrostatic nebuliser | Nanoparticles, <100 nm | <1 kg/d [28] |
The sizes of the synthesised nanoparticles depend on the ultrasound frequency [4][42][43], which determines the sizes of the aerosol droplets and the concentration of the dissolved salts in the precursor solution droplets. Due to vibrations of the ultrasound below the solution surface, the kinetic energy of the solution molecules increases rapidly. This causes small droplets to overcome the surface tension and break away from it. This effect, known as nebulisation (Figure 1), produces micron-sized aerosol droplets, which act as individual chemical reactors when subjected to thermal treatment [44][45]. Droplets in a size distribution from 1 to 15 µm are created with a high-frequency ultrasound (0.5–3 MHz) [46].
The generated droplets of the precursor solution are transported into the furnace with a carrier gas, where the synthesis stages of evaporation and droplet shrinkage, thermal decomposition and densification take place. Depending on the precursor salt composition and chemistry, a reaction gas may be included with the carrier gas, in order to promote the formation of pure metal or metal oxide nanoparticles. The process forms solid, nonporous nanoparticles with different amounts of aggregation, depending on the process conditions and parameters used (reaction temperature, gas flow, residence time, precursor selection and concentration).
3. Particle Formation with USP
3.1. Droplet-To-Particle Conversion Mechanism in USP
The liquid-to-solid and solid-to-solid conversion processes with USP can be described with the DTP mechanism. This mechanism, in general, consists of the formation of droplets, transportation of these droplets into a heating zone, evaporation of the solvent and thermal conversion of the solute into the final nanoparticles. As the droplet with dissolved material is being evaporated it shrinks, and, simultaneously, increases the mass fraction of the solute inside the droplet. The solute can begin to precipitate before uniform saturation is reached across the droplet because the solute diffusion is slower than the evaporation of the solvent. As the solid material is being precipitated on the droplet surface due to supersaturation, the liquid can become trapped in the centre. It then begins to evaporate through the newly formed surface crust (Figure 2). This slows down the evaporation rate.
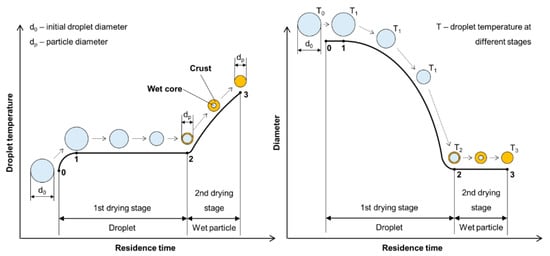
Solute precipitation in droplets has not been described by any theory in a quantitative manner. For a given solute, the supersaturation required for precipitation must be measured [4][48], and is a function of the exact composition of the solution (impurities act as precipitation sites). Because the rate of nucleation determines particle morphology, the evaporation determines particle morphology (evaporation depends on a number of factors, such as surrounding vapour pressure and temperature).
Intraparticle reactions, such as thermal decomposition, also occur in the aerosol before and after solvent evaporation, and can influence particle morphology. These were studied with thermogravimetric analysis, differential thermal analysis and differential scanning calorimetry [4]. The precursor characteristics determine whether hollow or porous particles are formed. Whether a precursor melts or not before reacting has a very distinct difference on the final nanoparticle morphology. The volume fraction of the solute also influences the formation of porous or hollow particles, as does a high reduction of volume because of reactions, shown in Figure 3.
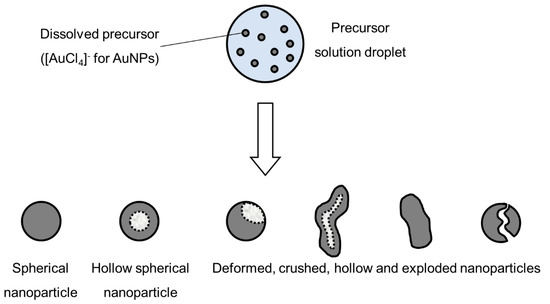
Figure 3. Types of nanoparticles that can be synthesised from an aerosol droplet, depending on the parameter conditions, adapted from [49].
3.2. Gas-To-Particle Conversion Mechanism in USP
The GTP formation mechanism generally follows the supersaturation of a gaseous species of the desired material, which causes nucleation and new particle formation. The particles can be created by chemical reactions of gaseous precursors, or by physical processes, such as cooling of hot vapour. The average particle diameter, total particle concentration, size distribution and particle morphology evolve along the aerosol reactor in two different modes. One mode is nucleation–condensation, where a monomer (a gas-phase molecule) is formed by a chemical reaction or a decrease in temperature until nucleation occurs. The saturation ratio increases, and growth of the monomer proceeds by condensation of the monomer onto the particles. When no collisions occur, they can remain nearly spherical through the aerosol reactor path. Surface reaction on the particles may also occur, promoting growth. Another mode is nucleation–coagulation, where particles are formed and their high concentration allows collisions and coalescence of particles, resulting in the growth of the particles. Both modes can be found in laboratory processes, while nucleation–condensation is usually not found in industrial systems.
In the nucleation–condensation mode, the morphology of the particles depends on collisions and coalescence. Particles coalesce by sintering after collisions in order to become spherical. The ratio of rates of collisions and coalescence (αc [4][50][51]) determines the morphology. In collision-limited growth (αc = ∞), the sintering rate is rapid relative to collisions, allowing for the formation of spherical particles between collisions. In sintering-limited growth (αc = 0) the particles exist as aggregates. Intermediate conditions (αc ≈ 1) are in existence in real situations, where the particle morphology is a function of parameters that control the sintering rate—temperature and material properties. The primary particle size is also a function of these parameters (Figure 4).
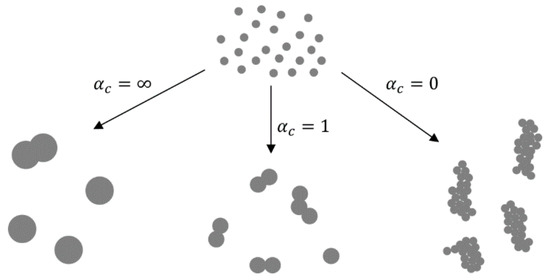
Figure 4. The ratios of collisions and coalescence with the Gas-To-Particle (GTP) mechanism and the resulting nanoparticle morphologies, adapted from [4][50].
When the aerosol droplet evaporates within the GTP mechanism, the solute is vapourised along with the solvent, resulting in the presence of the solute vapours and their partial vapour pressure. When saturation of vapours is reached, nucleation occurs, and growth of particles proceeds as described previously. Particles formed by the GTP conversion are typically smaller than the particles formed by the DTP mechanism.
4. Conclusions and Outlook
From the presented review the following conclusions can be summarised:
(1) USP is a potentially reliable method for the large scale synthesis of nanoparticles; (2) The size of obtained nanoparticles is connected with the droplet diameter and the initial concentration of the precursor solution; (3) In USP, in general, there are two conversion formation mechanisms of nanoparticles: DTP and GTP; (4) Through USP it is possible to synthesise AuNPs, particles decorated with nanoparticles (Ag/TiO2 and Au/TiO2), Au/Fe2O3 nanoparticles, photoluminescent Ag/(Y0.95 Eu0.05)2O3 nanoparticles and others, in the form of powder or in solution as a suspension; (5) Characterisation of nanoparticles synthesised through USP is complex, and is connected closely to the different techniques, such as SEM, STEM, TEM, EDX analysis and others, with the aim of obtaining information about size, morphology, chemical composition and possible functional properties of nanoparticles.
It is important to take into account that the USP is a very fast process where all the described processes take place in micrometres’ volume and in parts of seconds. For these reasons, the deviations of the proposed models are possible, and, based on this, synthesised nanoparticles are very often in a metastable state. The speed of the process and its reaction mechanisms also prevent real-time observations of the particle formation, while the many chemical and physical species present in the reaction tube (simultaneously present droplets of various sizes, vapours of solvents and salts) make modelling of the formation mechanisms difficult. This requires multiple optimisation experiments in order to obtain the desired particle characteristics, as the material properties are affected by several parameters. The formation models are, as such, derived from the characterisation of the finally obtained particles, while the connections between the parameters and the material properties are frequently not complete. More advanced characterisation technologies could alleviate this issue and provide more insight into the formation mechanisms, in order to reduce the number of optimisation experiments. This is especially important with upscaled USP devices, for reducing the costs of production set up.
An advantage of USP is its ability to produce complex morphologies and compositions in a one-pot synthesis method, with a continuous production, and is a reasonable development direction of the USP. This ability gives USP the potential for greater uptake in electronic, energy devices, solar cells and photocatalysis [52][53]. However, complex particles again increase the number of required optimisation steps and experiments to obtain the desired particle properties.
The rapid formation at elevated temperatures may also result in undesired properties of the final particles, such as crystallinity, composition or morphology. In some cases, subsequent thermal or chemical treatment of the particles produces the desired result, however, this decreases the advantage of using USP as a one-pot synthesis method, increasing particle production complexity and costs. In some cases, optimisation of the USP parameters and formation conditions can produce more favourable results without additional processing.
This entry is adapted from the peer-reviewed paper 10.3390/ma13163485
References
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289.
- Pattnaik, P. Surface plasmon resonance. Appl. Biochem. Biotechnol. 2005, 126, 79–92.
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294.
- Kodas, T.T.; Hampden-Smith, M.J. Aerosol Processing of Materials, 1st ed.; Wiley-VCH: New York, NY, USA, 1998; ISBN 978-0-471-24669-5.
- Debecker, D.P.; le Bras, S.; Boissière, C.; Chaumonnot, A.; Sanchez, C. Aerosol processing: A wind of innovation in the field of advanced heterogeneous catalysts. Chem. Soc. Rev. 2018, 47, 4112–4155.
- Sun, P.; Fang, Z.Z.; Zhang, Y.; Xia, Y. Review of the Methods for Production of Spherical Ti and Ti Alloy Powder. JOM 2017, 69, 1853–1860.
- Ma, J.; Wang, B.; Yang, Z.; Wu, G.; Zhang, J.; Zhao, S. Microstructure simulation of rapidly solidified ASP30 high-speed steel particles by gas atomization. Int. J. Min. Met. Mater. 2016, 23, 294–302.
- Lefebvre, A.H.; McDonell, V.G.; McDonell, V.G. Atomization and Sprays; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-12091-1.
- Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Direct aerosol synthesis of large-pore amorphous mesostructured aluminosilicates with superior acid-catalytic properties. Angew. Chem. Int. Ed. Engl. 2009, 48, 2784–2787.
- Buesser, B.; Pratsinis, S.E. Design of Nanomaterial Synthesis by Aerosol Processes. Ann. Rev. Chem. Biomol. Eng. 2012, 3, 103–127.
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112.
- Bore, M.T.; Rathod, S.B.; Ward, T.L.; Datye, A.K. Hexagonal Mesostructure in Powders Produced by Evaporation-Induced Self-Assembly of Aerosols from Aqueous Tetraethoxysilane Solutions. Langmuir 2003, 19, 256–264.
- Boissiere, C.; Grosso, D.; Chaumonnot, A.; Nicole, L.; Sanchez, C. Aerosol route to functional nanostructured inorganic and hybrid porous materials. Adv. Mater. 2011, 23, 599–623.
- Boissiere, C.; Grosso, D.; Amenitsch, H.; Gibaud, A.; Coupé, A.; Baccile, N.; Sanchez, C. First in-situ SAXS studies of the mesostructuration of spherical silica and titania particles during spray-drying process. Chem. Commun. 2003, 2798–2799.
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75.
- Wang, Y.-C.; Gunasekaran, S. Spectroscopic and microscopic investigation of gold nanoparticle nucleation and growth mechanisms using gelatin as a stabilizer. J. Nanopart Res. 2012, 14, 1–11.
- Lähde, A.; Koshevoy, I.; Karhunen, T.; Torvela, T.; Pakkanen, T.A.; Jokiniemi, J. Aerosol-assisted synthesis of gold nanoparticles. J. Nanopart Res. 2014, 16, 1–8.
- Tsai, S.C.; Song, Y.L.; Tsai, C.S.; Yang, C.C.; Chiu, W.Y.; Lin, H.M. Ultrasonic spray pyrolysis for nanoparticles synthesis. J. Mater. Sci. 2004, 39, 3647–3657.
- Okuyama, K.; Wuled Lenggoro, I. Preparation of nanoparticles via spray route. Chem. Eng. Sci. 2003, 58, 537–547.
- Suh, W.H.; Jang, A.R.; Suh, Y.-H.; Suslick, K.S. Porous, Hollow, and Ball-in-Ball Metal Oxide Microspheres: Preparation, Endocytosis, and Cytotoxicity. Adv. Mater. 2006, 18, 1832–1837.
- Patil, P.S. Versatility of chemical spray pyrolysis technique. Mater. Chem. Phys. 1999, 59, 185–198.
- Iskandar, F.; Mikrajuddin; Okuyama, K. In Situ Production of Spherical Silica Particles Containing Self-Organized Mesopores. Nano Lett. 2001, 1, 231–234.
- Oh, S.H.; Jo, M.S.; Jeong, S.M.; Kang, Y.C.; Cho, J.S. Hierarchical yolk-shell CNT-(NiCo)O/C microspheres prepared by one-pot spray pyrolysis as anodes in lithium-ion batteries. Chem. Eng. J. 2019, 368, 438–447.
- Jo, M.S.; Ghosh, S.; Jeong, S.M.; Kang, Y.C.; Cho, J.S. Coral-Like Yolk–Shell-Structured Nickel Oxide/Carbon Composite Microspheres for High-Performance Li-Ion Storage Anodes. Nanomicro Lett. 2019, 11, 3.
- Oh, S.H.; Kim, J.K.; Kang, Y.C.; Cho, J.S. Three-dimensionally ordered mesoporous multicomponent (Ni, Mo) metal oxide/N-doped carbon composite with superior Li-ion storage performance. Nanoscale 2018, 10, 18734–18741.
- Cho, J.S.; Ju, H.S.; Lee, J.-K.; Kang, Y.C. Carbon/two-dimensional MoTe2 core/shell-structured microspheres as an anode material for Na-ion batteries. Nanoscale 2017, 9, 1942–1950.
- Cho, J.S.; Lee, S.Y.; Lee, J.-K.; Kang, Y.C. Iron Telluride-Decorated Reduced Graphene Oxide Hybrid Microspheres as Anode Materials with Improved Na-Ion Storage Properties. ACS Appl. Mater. Interfaces 2016, 8, 21343–21349.
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072.
- Hernandez, R.T.L.; Escalona, A.; Granados, S.J.; Sánchez, A.; Orozco, S. Ultrasonic spray pyrolysis synthesis of Al, Zr, and Ti oxides multishell. J. Phys. Conf. Ser. 2019, 1221, 012026.
- Tran, M.N.; Cleveland, I.J.; Aydil, E.S. Resolving the discrepancies in the reported optical absorption of low-dimensional non-toxic perovskites, Cs3Bi2Br9 and Cs3BiBr6. J. Mater. Chem. 2020, 8, 10416–10421.
- Rudolf, R.; Friedrich, B.; Stopić, S.; Anžel, I.; Tomić, S.; Čolić, M. Cytotoxicity of gold nanoparticles prepared by ultrasonic spray pyrolysis. J. Biomater. Appl. 2012, 26, 595–612.
- Dokić, J.; Rudolf, R.; Tomić, S.; Stopić, S.; Friedrich, B.; Budic, B.; Anzel, I.; Colić, M. Immunomodulatory properties of nanoparticles obtained by ultrasonic spray pirolysis from gold scrap. J. Biomed. Nanotechnol. 2012, 8, 528–538.
- Tomić, S.; Đokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I.; et al. Size-Dependent Effects of Gold Nanoparticles Uptake on Maturation and Antitumor Functions of Human Dendritic Cells In Vitro. PLoS ONE 2014, 9, e96584.
- Shariq, M.; Friedrich, B.; Budic, B.; Hodnik, N.; Ruiz-Zepeda, F.; Majerič, P.; Rudolf, R. Successful Synthesis of Gold Nanoparticles through Ultrasonic Spray Pyrolysis from a Gold(III) Nitrate Precursor and Their Interaction with a High Electron Beam. Chem. Open 2018, 7, 533–542.
- Golub, D.; Ivanič, A.; Majerič, P.; Tiyyagura, H.R.; Anžel, I.; Rudolf, R. Synthesis of Colloidal Au Nanoparticles through Ultrasonic Spray Pyrolysis and Their Use in the Preparation of Polyacrylate-AuNPs’ Composites. Materials 2019, 12.
- Bogovic, J.; Rudolf, R.; Friedrich, B. The Controlled Single-Step Synthesis of Ag/TiO2 and Au/TiO2 by Ultrasonic Spray Pyrolysis (USP). JOM 2015.
- Alkan, G.; Rudolf, R.; Bogovic, J.; Jenko, D.; Friedrich, B. Structure and Formation Model of Ag/TiO2 and Au/TiO2 Nanoparticles Synthesized through Ultrasonic Spray Pyrolysis. Metals 2017, 7, 389.
- Jung, D.S.; Park, S.B.; Kang, Y.C. Design of particles by spray pyrolysis and recent progress in its application. Korean J. Chem. Eng. 2010, 27, 1621–1645.
- Stopic, S.; Rudolf, R.; Bogovic, J.; Majerič, P.; Čolić, M.; Tomić, S. Synthesis of Au nanoparticles prepared by ultrasonic spray pyrolysis and hydrogen reduction. Mater. Tehnol. 2013, 47, 577–583.
- Majerič, P.; Jenko, D.; Budič, B.; Tomić, S.; Čolić, M.; Friedrich, B.; Rudolf, R. Formation of Non-Toxic Au Nanoparticles with Bimodal Size Distribution by a Modular Redesign of Ultrasonic Spray Pyrolysis. Nanosci. Nanotechnol. Lett. 2015, 7, 920–929.
- Manivasakan, P.; Karthik, A.; Rajendran, V. Mass production of Al2O3 and ZrO2 nanoparticles by hot-air spray pyrolysis. Powder Technol. 2013, 234, 84–90.
- Messing, G.L.; Zhang, S.-C.; Jayanthi, G.V. Ceramic Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726.
- Xiong, Y.; Kodas, T.T. Droplet evaporation and solute precipitation during spray pyrolysis. J. Aerosol Sci. 1993, 24, 893–908.
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059.
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Mdleleni, M.M.; Wong, M. Acoustic Cavitation and Its Chemical Consequences. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 1999, 357, 335–353.
- Bogović, J.; Schwinger, A.; Stopic, S.; Schröder, J.; Gaukel, V.; Schuchmann, H.P.; Friedrich, B. Controlled droplet size distribution in ultrasonic spray pyrolysis. Metall 2011, 65, 455–459.
- Mezhericher, M.; Levy, A.; Borde, I. Heat and mass transfer of single droplet/wet particle drying. Chem. Eng. Sci. 2008, 63, 12–23.
- Hileman, O.E., Jr. Precipitation from homogeneous solution applied to the drop technique for the study of nucleation. Talanta 1967, 14, 139–140.
- Draper, N.D. Investigation of Surface-Potential Controlled Nucleation Using an Acoustic Levitation Apparatus. Ph.D. Thesis, Department of Chemistry, Simon Fraser University, Burnaby, BC, Canada, 2012.
- Wu, M.K.; Windeler, R.S.; Steiner, C.K.R.; Börs, T.; Friedlander, S.K. Controlled Synthesis of Nanosized Particles by Aerosol Processes. Aerosol Sci. Technol. 1993, 19, 527–548.
- Kruis, F.E.; Kusters, K.A.; Pratsinis, S.E.; Scarlett, B. A Simple Model for the Evolution of the Characteristics of Aggregate Particles Undergoing Coagulation and Sintering. Aerosol Sci. Technol. 1993, 19, 514–526.
- Mueller, R.; Mädler, L.; Pratsinis, S.E. Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem. Eng. Sci. 2003, 58, 1969–1976.
- Rahemi Ardekani, S.; Sabour Rouh Aghdam, A.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631.
