In light of expanding incidences of keratinocyte carcinoma (KC) with many patients developing multiple tumors, the demand for new treatment modalities is high. With the approval of cemplimab for locally advanced and metastasizing basal cell carcinoma and squamous cell carcinoma, KC is now included as an indication for systemic immunotherapy. At present, however, systemic KC therapy remains limited by the severe side effects associated with treatment. Immunotherapy might be more broadly applied if locally administered. Localized to the skin, KCs are easily accessible to topical drugs and physical interventions such as laser. There is an increasing appreciation of lasers’ potential to activate an immune response. Further enhancement of the laser-based immune activation might be obtained by combining laser and immunotherapeutic agents, known as laser immunotherapy.
1. Introduction
In oncology, the role of the immune system in cancer prevention and control is well-recognized, and the introduction of systemic immunotherapeutics has revolutionized clinical cancer treatment. Keratinocyte carcinomas (KCs), however, differ from many cancer types in that most tumors remain localized, with low metastasizing potential. Thus, only few patients with aggressive disease are candidates for systemic immunotherapy, and associated treatment toxicity remains a major limiting factor. If locally administered, immunotherapy might be more broadly applied to treating KCs. The cutaneous localization of KC renders this cancer type easily accessible to topically applied drugs, as well as physical interventions such as laser.
Dermatologists were among the first medical specialists to incorporate lasers in medicine, where treatment of skin cancer was an early indication of interest [
1]. For decades, the focus of laser-based treatment of KC, comprising basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), has been on the modality’s tumor destructive effects and closure of vessel supply [
2,
3]. Clinical application of laser therapy for KC has since broadened with introduction of fractional laser-assisted drug delivery, a technique which enhances topical delivery of drugs through the upper skin layers [
4,
5]. Now, beyond causing physical tumor destruction and facilitation of cutaneous drug distribution, there is an increasing appreciation of lasers’ potential to activate an anti-tumoral immune response through controlled tissue injury. Ideally suited to treat tumors freely accessible on the skin, lasers’ impact on local immune environments might be harnessed to treat KC as illustrated in
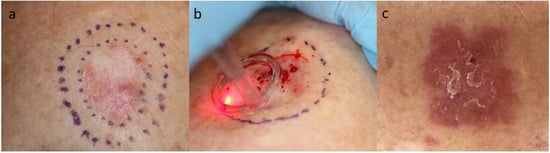
Figure 1 where a BCC is treated with an ablative fractionated laser (AFL) (
Figure 1).
Figure 1. Photos of a BCC prior to AFL (a); upon intervention with AFL (b) and 1 week after AFL (c). The photo in the middle (b) shows the immediately generated laser channels (white grid in the area of the laser beam) on the skin. The latter photo (c) shows erythema of the AFL-treated area with resulting impact on the skin. Ultrapulse CO2-laser (10,600 nm, Lumenis, Santa Clara, CA, USA), 100 mJ/mb, 5% density). Department of Dermatology, Copenhagen University Hospital, Bispebjerg. Photos shown with patient’s consent.
Termed laser immunotherapy (LIT), the concept of combining immune and laser therapy, has multiple potential advantages, including enabling topical delivery of immunological agents, as well as laser-based amplification of immunotherapeutic agents. This work presents rationales for use of immune-based treatment of KC and examines the current status of KC immunotherapy. While the term KC includes both BCC and SCC, it is important to state that these tumors differ both in terms of clinical presentation and aggressiveness as well as in their biological evolution. Impairment of the sonic hedgehog pathway plays a key role in BCC pathogensis, the most prevalent of the KCs. BCC display very low metastazising potential, while SCC metastazises in 4–6% of cases [
6]. While surgical excision and radiofrequency are accepted treatments for both BCC and SCC, topical immunotherapy is restricted to BCC. Systemic immunotherapy, however, is now approved for both tumors where conventional treatment is inadequate due to severe disease.
2. Rationales for Immunotherapy in Keratinocyte Carcinoma
The immune system’s ability to recognize and eliminate transformed malignant cells is well established. Improved understanding of tumor pathophysiology and the role of the immune system in tumor control, has led to the development of systemic immunotherapy; one of the most important breakthroughs in modern medicine for treatment of various aggressive cancers, including malignant melanoma (MM) [
7].
In the context of KC treatment, two biomolecular rationales support the use of immune check point inhibitors: (1) the presence of programmed death-1 (PD1) on T-cells or programmed death ligand-1 (PD-L1) on cancer cells and suppressive immune cells in tumor tissue and (2) the high mutational burden of KC. Currently, these markers are considered to be among the most valid general predictors of response of immune check point inhibition.
Two larger immunohistochemical (IHC) studies focusing on PD1/PD-L1 in BCC showed positive staining in the majority of tumor cells and tumor infiltrating lymphocytes (TILs), indicating a potential for response [
8,
9]. It should be noted however that a phase 2 study on cemiplimab (anti-PD1) in SCC showed clinical responses irrespective of baseline PD-L1 status [
10]. More specific focus on a subgroup of PD1 positive TILs, namely regulatory T-cells (T-regs), is now appreciated to be a predictor of treatment response. Accordingly, melanoma patients who demonstrated a rapid decline in circulating PD1-positive T-regs upon anti-PD1 treatment were at reduced risk for disease progression [
11]. A study on BCC tumor environment has revealed increased T-reg/CD3 ratio in the tumor microenvironment, a feature that is suggested to play a role in tumor escape and further supports the concept of immune checkpoint inhibition in KC management [
12]. Whether these T-regs are PD1 positive remains to be elucidated but might prove important, since PD1 signaling is involved in T-reg homeostasis. Interestingly, a previous preclinical study has shown PD1–deficient T-regs to possess increased immunosuppressive activity compared with PD1–intact T-regs [
13], indicating that lack of PD-1 signaling enhances the immunosuppressive function of T-regs. Likewise, murine PD-1 deficient T-regs have been shown to be more proliferative and immunosuppressive compared with PD1 intact T-regs [
14].
The second emerging biomarker predicting the outcome of checkpoint inhibitors is the tumor mutational burden (TMB) [
15]. TMB is a quantitative measure of the number of gene mutations inside cancer cells and is an indirect measure of tumor-derived neoantigens. It is hypothesized that the higher the number of neoantigens within a tumor, the higher probability of target of recognition exists within the tumor for anti-tumor immune response. Genome studies have revealed that KCs have the highest mutational burden of all human cancers, providing another argument for KC immunotherapy [
16]. A case series including eight patients with metastatic BCC, four of whom received anti-PD1, presenting the genomic correlates on advanced/metastatic BCC treated with anti-PD1 revealed biological features (high TMB; PD1/PD-L1 amplification) predictive of immunotherapy benefit [
17].
The role of the immune system in KC development and maintenance is underscored by substantially higher BCC and SCC rates in immunosuppressed versus immunocompetent individuals [
18,
19].
3. Keratinocyte Carcinoma Immunotherapy: Current Status
The clinical development of immune checkpoint inhibitors has drastically expanded within the last decade, both in terms of new drugs and perhaps more markedly, cancer indications [
7,
20]. Cemiplimab, a PD1 inhibitor, is the first immune check point inhibitor approved for the treatment of KC of the skin. The drug is authorized for the treatment of locally advanced and metastasizing SCC. In these tumors, cemiplimab demonstrates durable, clinically significant efficacy with an objective response in 44% [
10] and 47%, respectively, [
21] and an acceptable safety profile. Most recently, cemiplimab was approved for locally advanced and metastatic BCCs either previously treated with a hedgehog pathway inhibitor, or in patients where hedgehog pathway inhibitor is inappropriate. The overall response rate of cemiplimab appears lower for BCCs than SCCs reported in one study as 21% (6/28) in metastatic BCC patients, with no complete responses. In patients with locally advanced BCC, the objective response rate is 29% (24/84), with 6% (5/84) complete responders (trial ID: R2810-ONC-1620).
In addition to cemiplimab studies, evidence of a clinically relevant potential for anti-PD1 treatment against KC has been reported in patients with MM on anti-PD1 treatment. In that population, lower incidences of BCC compared with patients with MM not receiving anti-PD1 was shown. No difference in SCC-incidence, however, was found. Given the aforementioned differences in response rates to cemiplimab for BCC and SCC, the lack of impact regarding SCC incidence is surprising. This could reflect overall lower incidences of SCC compared with BCC, resulting in small sample size [
22]. Additionally, patients with metastasizing BCC have been found to show partial or near-complete response to anti-PD1 in five case reports [
23,
24,
25,
26,
27]. Most recently, a study on the effect of PD-L1-directed vaccination in 10 patients with BCC was published. Vaccinations resulted in vaccine-specific immune responses detectable in blood samples from nine of 10 patients and in skin samples from five of nine patients, suggesting that a PD-L1 vaccine might be effective against some BCCs with minor adverse reactions [
28].
Systemic immunotherapy comes with a significant risk of side effects, the seriousness of which must be outweighed by cancer aggressiveness. Since most KCs are localized skin tumors often arising in elderly patients with comorbidities, systemic immunotherapy is reserved for a minority with locally advanced or metastasized disease. In comparison, topical therapy is usually associated with a more tolerable side effect profile. Imiquimod is an approved topical immunotherapy for KC that is associated with markedly fewer systemic side effects. The agent’s use is however restricted to superficial BCC [
29] and actinic keratoses. Imiquimod is a toll-like receptor (TLR) agonist that binds to TLR 7 and 8 present on innate immune cells to produce anti-viral and anti-tumoral effects. The drug stimulates plasmacytoid dendritic cells to release INF-α [
30] and leads to influx of CD8 positive T-cells, B cells as well as macrophages [
31]. Seeking to broaden the treatment indication of imiquimod, combination treatment with physical tumor treatment has been introduced; imiquimod combined with cryotherapy showed promising efficacy for BCC and in situ SCC, with combination therapy being more effective than either treatment alone [
32,
33]. Going forward, combined imiquimod with laser may exploit not only laser’s destructive effects, but also the modality’s potential for immune activation, conceivably leading to enhanced immunotherapeutic effects.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13215405