There is national and international growing concern on human health risk-benefit related to seafood exposure. The information derived from these studies is controversial, has brought about more questions than answers and the quest for irrefutable evidence is an on-going reality that can be perceived as a continuous process that demands a great deal of time, debate to select appropriate outcomes and most importantly solid experimental proof to reach a final agreement between the various bodies, a vital aspect for proposing rational approaches to risk-benefit assessment.
- Risk-benefit assessments
- Nutrition
- Biomarkers
- Fatty acids
- Eicosanoids
Pedro Araujo
Institute of Marine Research (IMR), PO Box 1870 Nordnes, N-5817 Bergen, Norway
Dietary risk-benefit assessments: controversies
Dietary exposure studies for evaluating seafood safety and food health aspects are currently based either on a standard risk-benefit model or on well-established biomarkers that fully explain both toxicological and nutritional effects. The lack of a common model and different nutritional recommendations have triggered some controversy wherein research groups, governmental and environmental agencies, health organizations and international committees which have pinpointed some doubts regarding data quality, selected outcomes or the applicability of findings to countries beyond the study population [1, 2].
A published systematic review on the benefits of omega-3 fatty acids (ω-3 FA) concluded that long chain and shorter ω-3 FA did not have a clear effect on total mortality, combined cardiovascular events, or cancer [3]. The relationship between the consumption of fish and intake of long-chain ω-3 FA, and the risk of coronary heart mortality in men and women free of cardiovascular diseases indicated that the protective effect of fish consumption against cardiovascular diseases is observed in women but it was not found in men [4]. In marked contrast to these results, it has been reasserted the remarkable cardioprotective effects of longer-chain ω-3 FA from marine sources, suggesting that consumption should be increased in the diet to decrease cardiovascular risk significantly [5, 6]. Despite their positive evidence, these authors, in line with the U.S Food and Drug Administration (FDA), suggested caution in the consumption of certain fish species depending on their levels of environmental pollutants. The FDA maintains that the consumption of fish should be limited in pregnant women and young children as it may contain high levels of mercury [7]. The Environmental Working Group has alleged that they found a non-published FDA report that talks of the likely benefits of fish consumption in adults and children alike. The document in question reads, "The net effect on fetal neurodevelopment from eating commercial fish containing methyl mercury is not necessarily adverse and could in fact be beneficial" [8]. It has been reported that salmon and trout sold in markets could be consumed regularly to achieve optimal nutritional benefits from ω-3 FA, without incurring significant contaminant related health risks [9]. It seems that while there is a significant amount of data showing health benefits of increased fish consumption, there are conflicting reports about the cardiovascular risks of mercury in seafood [10]. A peremptory challenge that further studies should solve is whether or not the role attributed to ω-3 FA intake, mainly from fish consumption, is indeed as beneficial as considered until now, especially due to the high levels of ω-3 polyunsaturated FA (PUFA), which may protect against several adverse health effects such as coronary heart disease, stroke, pain, inflammation process and mortality [11].
Dietary risk-benefit assessments: associated problems
The evaluation of historical data along with their different supporting experimental approaches are invaluable research tools to establish risk-benefit studies provided that collection, handling and storage rely on similar procedures. For example, the Norwegian Biobank Act published in 2003 [12] regulates the collection and storage of biological material. However procedural uniformity aspects have not been addressed in many national and overseas legislations, causing divergent collection protocols that might impact the outcomes of established biobanks and leading to an incorrect association between exposure, pathology and toxicity. For instance, although several studies have indicated the importance of adding antioxidantans and cyclooxigenase inhibitors to blood samples to preserve the identity of the FA composition for at least 1 year and to prevent the in vitro formation of inflammatory biomarkers respectively [13, 14], a biobank of some renown has collected more than 380,000 plasma samples without antioxidants or cyclooxigenase inhibitors to be stored for up to 100 years and to be submitted periodically to gas chromatography (GC) for FA analysis [15].
The lack of butylated hydroxytoluene (BHT), an antioxidant generally used during blood collection, has been reported to cause a decrease of approximately 40 % for eicosapentaenoic acid (EPA; 20:5ω-3), docosahexaenoic acid (DHA; 20:6ω-3) and arachidonic acid (AA; 20:4ω-6) after 17 weeks storage [16]. A Recent study [17] concluded that addition of BHT to blood specimens is essential to ensure the stability of the FA in the samples up to a year. Similarly, lack of indomethacin, a cyclooxigenase inhibitor added to prevent the in vitro formation of inflammatory biomarkers, is essential if the aim of the study is to determine pro-inflammatory biomarkers such as prostaglandins. For example, parallel plasma samples with and without indomethacin from patients suffering from various gastrointestinal pathologies were collected and revealed a significant correlation between pain amelioration and reduction of PGE2 after exposure to seal oil in samples containing indomethacin [18]. The same set of samples without indomethacin did not show any consistent reduction in PGE2 levels. In addition, several studies on the beneficial role of longer-chain ω-3 FA, mainly DHA and EPA, from marine sources in cardiovascular health diseases have suggested that the modulation ω-3 FA and methylmercury (MeHg), an organometallic contaminant generally associated with stroke risk and neurotoxicity, poses a potential health risk to consumers [4, 19, 20].
In general, studies on the assessment of risk-benefit related to seafood have not considered the effect of ω-6 FA present in the samples along with their metabolites and their relation to ω-3 FA and/or contaminants. The ω-6 AA through the action of cyclooxygenases (COX) and lipooxygenase (LOX) enzymes is converted into eicosanoids with high biological activity known as the 2-series prostanoids and 4-series leukotrienes. Studies performed on fish and mammalian have suggested that eicosanoids such as PGE2 and leukotriene B4 (LTB4) play a primordial role in several pathological conditions such as inflammation [21, 22] and pain [23]. Experimental studies have demonstrated that the relative abundance of AA is an important factor in eicosanoids potency and mode of action [24]. Studies in Norway on the effect of seal oil, a rich source of ω-3 PUFA, on patients with Crohn’s disease and ulcerative colitis revealed a remarkable alleviation in both disease activities after supplementing their diet with seal oil administered nasoduodenally [25-27]. It has been demonstrated that the incorporation of dietary ω-3 PUFA in patients with rheumatoid arthritis alleviates inflammatory diseases by reducing the contribution of pro-inflammatory metabolites PGE2 and LTB4 and promoting anti-inflammatory metabolites PGE3 and LTB5 [28]. Besides the importance of ω-3 PUFA in the diets of patients suffering from inflammatory chronic diseases, the presence of ω-6 PUFA can also contribute to suppress the production of inflammatory lipid mediators. Dihomo-γ-linolenic acid (DGLA; 20:3ω-6) is a precursor to the 1-series prostaglandins, such as the anti-inflammatory PGE1. In addition, a study by Johns Hopkins scientists has revealed that PGE2 can provide some protection against brain cell death (ScienceDaily., 2005) [29]. These observations suggest that there should be a healthy balance between ω-3 and ω-6 PUFA in the diet.
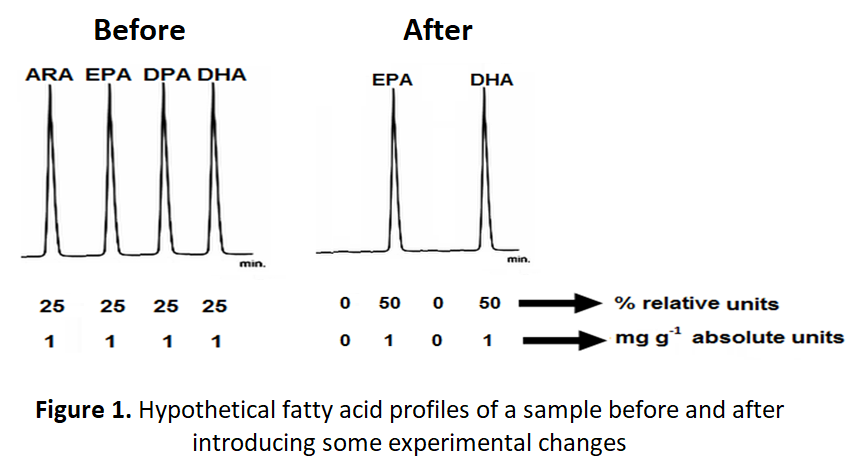
Dietary exposure studies evaluating FA profiles should also pay special attention to the impact of the measurement units (e.g. absolute or relative) on the final conclusions. Absolute (mg g-1) and relative (%) amounts might give conflicting results when variations in the experimental conditions are evaluated. Figure 1 recreates the hypothetical chromatograms of a blood specimen from a patient that is measured before and after introducing an experimental change (e.g. temperature, time, a magic pill that reduces the ω-6 levels). The chromatograms exhibit four and two chromatographic peaks before and after the change, respectively. The chromatographic peaks are identified as ARA, EPA, docosapentaenoic acid (DPA; 22:6ω-3) and DHA.
The conclusion derives from the analysis of the relative amounts (%) indicated that the introduced change caused a remarkable 100% increase in EPA and DHA (from 25 to 50%). However, the conclusion from absolute amounts (mg g-1) clearly indicates that the introduced change has a detrimental effect on ARA and DPA but not on EPA and DHA which concentrations remain constant throughout the analyses. Some studies have used the relative levels of ARA and DHA in maternal milk collected from women in Australia, Canada, Chile, China, Japan, Mexico, Philippines, United Kingdom, and United States and concluded that the levels of the ARA were comparable, while the levels of DHA varied between countries [30]. It is beyond the scope of the present communication to judge how trustworthy are the findings of this comparative study, however dietary exposure studies for evaluating seafood safety and food health aspects should be cautious about the reliability of conclusions derived from using relative units. The best approach is to use either absolute units or both units.
Despite the wide spectrum of effects activated by the ω-3 and ω-6 fatty acid metabolites, they have been ignored in risk-benefit studies. It is surprising that although ω-6 AA alone is responsible for the production of over 100 different metabolites with different physiological functions, most of risk-benefit studies did not pay attention to the implication of at least few of these metabolites in their outcomes.
Conclusion
The current literature revealed that in general dietary risk-benefit assessments are characterized by incomplete or inadequate measures of potential risk factors which may yield systematic under-estimates of disease associations; incomplete or inadequate measures of confounding factors; and/or retrospective case-control designs in which the disease itself may influence risk factor levels (UK Biobank., 2006) [31]. The inclusion of potential and meaningful biomarkers and the assessment of factors that can affect the stability of the traditional biomarkers are important aspects that have not been considered in risk-benefit studies yet and that should urgently be addressed to yield comprehensive and reliable associations between exposure and pathology.
References
- Mozaffarian D., Rimm E.B. (2006) Fish intake, contaminants, and human health: evaluating the risks and the benefits. Journal of the American Medical Association 296:1885-1899.
- European Food Information Council (2006) Fish benefits outweigh risks, conclude two studies. http://www.eufic.org/page/en/show/latest-science-news/fftid/fishcardiovascular-food-contaminants/
- Hooper R.L., Thompson R.L., Harrison, R.A., Summerbell C.D., Ness A.R., Moore H.J., Worthington H.V., Durrington P.N., HigginsP.T., Capps N.E., Riemersma R.A., Ebrahim S.B.J., Smith G.D. (2006) Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. British Medical Journal 332:752–760.
- Järvinen R., Knekt P., Rissanen H., Reunanen A. (2006) Intake of fish and long-chain n-3 fatty acids and the risk of coronary heart mortality in men and women. British Journal of Nutrition 95:824–829.
- Psota T.L., Gebauer S.K., Kris-Etherton P. (2006) Dietary omega-3 fatty acid intake and cardiovascular risk, American Journal of Cardiology 98:3i–18i.
- Engler M.M., Engler M.B. (2006) Omega-3 fatty acids: role in cardiovascular health and disease, Journal of Cardiovascular Nursing 21:17–24.
- FDA & EPA. (2004) What you need to know about mercury in fish and shellfish - Advice for women who might become pregnant women who are pregnant nursing mothers young children. Advisory EPA-823-F-04-009.
- Washington J.P. (2008) FDA re-evaluates recommendation on fish consumption. http://www.themedguru.com/articles/fda_re_evaluates_recommendation_on_fish_consumption-86119655.html
- Dewailly E., Ayotte P., Lucas M., Blanchet C. (2007) Risk and benefits from consuming salmon and trout: A Canadian perspective. Food and Chemical Toxicology 45:1343-1348
- Levenson C.W., Axelrad D.M. (2006) Too much of a good thing? Update on fish consumption and mercury exposure. Nutrion Reviews 64:139–145.
- Domingo J.L. (2007). Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environment International 33:998-993.
- Lovdata (2003). Lov om behandlingsbiobanker (behandlingsbiobankloven). Act 2003-02-21-12. http://www.lovdata.no/all/nl-20030221-012.html
- Otto S.J., Foreman-van Drongelen M.M., Von Houwelingen A.C., Hornstra, G. (1997) Effects of storage on venous and capillary blood samples: the influence of deferoxamine and butylated hydroxytoluene on the fatty acid alterations in red blood cell phospholipids. European Journal of Clinical Chemistry and Clinical Biochemistry 35:907–913.
- Di Marino L., Maffettone A., Cipriano P., Celentano E., Galasso R., Iovine C., Berrino F., Panico, S. (2000) assay of erythrocyte membrane fatty acids. effects of storage time at low temperature. International Journal of Clinical Laboratory Research 30:197-202.
- Rønningen K.S., Paltiel L., Meltzer H.M., Nordhagen R., Lie K.K, Hovengen R., Haugen M., Nystad W., Magnus P., Hoppin J.A. (2006) The biobank of the Norwegian mother and child cohort study: a resource for the next 100 years. European Journal of Epidemiology, 21:619-625.
- Magnusardottir A.R., Skuladottir G.V. (2006) Effects of storage time and added antioxidant on fatty acid composition of red blood cells at −20°C. Lipids 41: 401-404.
- Araujo P., Bjørkkjær T., Frøynad L., Waagbø R. (2018) Effect of storage time, temperature, antioxidant and thawing on fatty acid composition of plasma, serum and red blood cells - A pilot biobank study. Clinical Biochemistry, 52:94-105
- Bjørkkjær T., Araujo P., Madland T.M., Berstad A., Frøyland L. (2009) A randomized double blind comparison of short-term duodenally administrated whale and seal blubber oils in patients with inflammatory bowel disease and joint pain. Prostaglandins, Leukotrienes and Essential Fatty Acids 81:425-432.
- Chan H.M., Egeland G.M. (2004) Fish consumption, mercury exposure, and heart diseases. Nutrition Reviews 62:68-72.
- Myers G.J., Davidson P.W., Strain J.J. (2007) Nutrient and methyl mercury exposure from consuming fish. The Journal of Nutrition 137:2805-2808.
- Archer C.B., Page C.P., Juhlin L., Morley J., MacDonaldM. (1978) Delayed synergism between leukotriene B4 and prostaglandin E2 in human skin. Prostaglandins 33:799-805.
- Martens L.G., Lock E.J., Fjelldal P.G., Wargelius A., Araujo P., Torstensen B., Witten E., Hansen T., Waagbø R., Ørnsrud R. (2010) Dietary fatty acids and inflammation in the vertebral column of Atlantic salmon (Salmo salar) smolts: a possible link to spinal deformities. Journal of Fish Diseases 33:957-972.
- Heinricher M.M., Martenson M.E., Neubert M.J. (2004) Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain 110:419-426.
- Mustafa T., Srivastava K.C. (1989) Prostaglandins (eicosanoids) and their role in ectothermic organisms. Advances in Comparative & Environmental Physiology 5:157–207.
- Arslan G., Brunborg L.A., Froyland L., Brun J.G., Valen M., Berstad A. (2002) Effects of duodenal seal oil administration in patients with inflammatory bowel disease. Lipids 37:935-940.
- Bjørkkjær T., Brunborg L.A., Arslan G., Lind R.A., Brun J.G., Valen M., Klementsen B., Berstad A., Frøyland L. (2004) Reduced joint pain after short-term duodenal administration of seal oil in patients with inflammatory bowel disease: comparison with soy oil. Scandinavian Journal of Gastroenterology 39:1088-1094.
- Bjorkkjaer T, Brun J.G., Valen M., Arslan G., Lind R., Brunborg L.A., Berstad A., Froyland L. (2006) Short-term duodenal seal oil administration normalised n-6 to n-3 fatty acid ratio in rectal mucosa and ameliorated bodily pain in patients with inflammatory bowel disease. Lipids in Health and Disease 5:1-6.
- James M.J., Cleland L.G. (1997) Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Seminars in Arthritis and Rheumatism 27:85-97.
- Johns Hopkins Medical Institutions (2005) Researchers reveals how certain chemicals protect the brain against cell damage. ScienceDaily, 21 November 2005. https://www.sciencedaily.com/releases/2005/11/051121163004.htm
- Yuhas R., Pramik K., Lien E.L. (2006) Human milk fatty acid composition from nine countries varies most in DHA. Lipids 41:851-858.
- UK Biobank. (2006) Protocol for a large-scale prospective epidemiological resource. Protocol No: UKBB-PROT-09-06.