Tomato spotted wilt virus (TSWV) is one of the most destructive diseases affecting tomato (Solanum lycopersicum) cultivation and production worldwide. As defenses against TSWV, natural resistance genes have been identified in tomato, including Sw-1a, Sw-1b, sw-2, sw-3, sw-4, Sw-5, Sw-6, and Sw-7. However, only Sw-5 exhibits a high level of resistance to the TSWV. Thus, it has been cloned and widely used in the breeding of tomato with resistance to the disease. Due to the global spread of TSWV, resistance induced by Sw-5 decreases over time and can be overcome or broken by a high concentration of TSWV. How to utilize other resistance genes and identify novel resistance resources are key approaches for breeding tomato with resistance to TSWV. In this review, the characteristics of natural resistance genes, natural resistance resources, molecular markers for assisted selection, and methods for evaluating resistance to TSWV are summarized. The aim is to provide a theoretical basis for identifying, utilizing resistance genes, and developing tomato varieties that are resistant to TSWV.
- tomato
- tomato spotted wilt virus
- resistance gene
- resistant resource
- molecular marker-associated breeding
1. Introduction
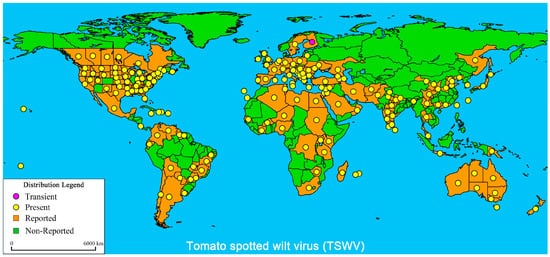
2. TSWV Symptoms in Tomato Plants

3. Methods for Identifying Resistance to TSWV in Tomato
3.1. Mechanical Inoculation
3.2. Thrip Inoculation
4. Natural Resources Resistant to the TSWV in Tomato
5. Natural Genes Resistant to TSWV in Tomato
5.1. Sw-1a and Sw-1b
5.2. Sw-2, Sw-3, and Sw-4
5.3. Sw-5
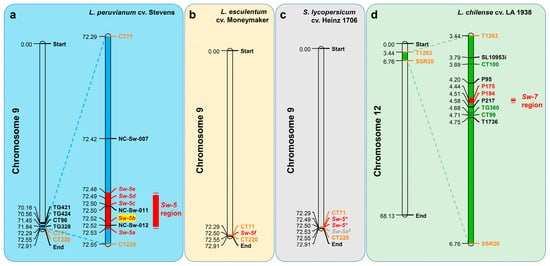
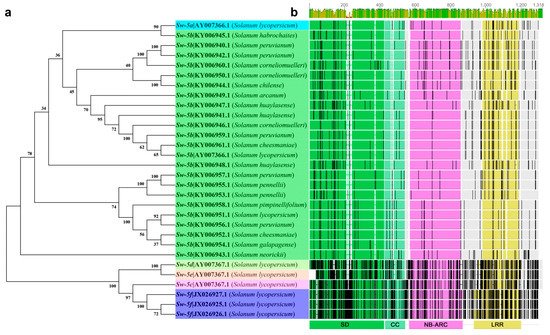
5.4. Sw-6
5.5. Sw-7
6. Molecular Markers for Resistance to TSWV in Tomato
7. Mechanism of Natural Resistance to the TSWV in Tomato
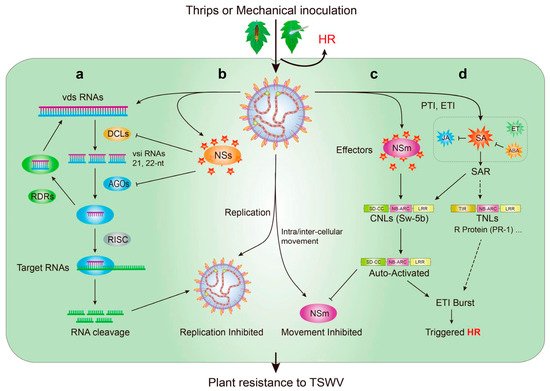
8. Challenge and Prospects
9. Conclusions
References
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT—Crops and Livestock Products. Latest Update: 15 September 2021. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 28 September 2021).
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging viral diseases of tomato crops. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef]
- Nilon, A.; Robinson, K.; Pappu, H.R.; Mitter, N. Current status and potential of RNA interference for the management of Tomato spotted wilt virus and thrips vectors. Pathogens 2021, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- De Haan, P.; Kormelink, R.; de Oliveira Resende, R.; van Poelwijk, F.; Peters, D.; Goldbach, R. Tomato spotted wilt virus L RNA encodes a putative RNA polymerase. J. Gen. Virol. 1991, 71, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.; Quadt, R.; Choi, T.J.; Ahlquist, P.; German, T. An RNA-dependent RNA-polymerase activity associated with virions of Tomato spotted wilt virus, a plant-infecting and insect-infecting bunyavirus. Virology 1995, 207, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.-S.; Jang, S.-W.; Jeon, Y.-H. Complete genome sequence of Tomato spotted wilt virus from paprika in Korea. Int. J. Plant Pathol. 2013, 2, 121–136. [Google Scholar] [CrossRef]
- Nagata, T.; Inoue-Nagata, A.K.; Prins, M.; Goldbach, R.; Peters, D. Impeded thrips transmission of defective Tomato spotted wilt virus isolates. Phytopathology 2000, 90, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kwon, S.-Y.; Kim, S.T. An insight into the Tomato spotted wilt virus (TSWV), tomato and thrips interaction. Plant Biotechnol. Rep. 2018, 12, 157–163. [Google Scholar] [CrossRef]
- Storms, M.M.H.; Kormelink, R.; Peters, D.; VanLent, J.A.W.M.; Goldbach, R.W. The nonstructural NSm protein of tomato spotted wilt virus induces tubular structures in plant and insect cells. Virology 1995, 214, 485–493. [Google Scholar] [CrossRef]
- Sonoda, S.; Tsumuki, H. Analysis of gene sequences for the nucleocapsid protein from Tomato spotted wilt virus for promoting RNA-mediated cross-protection using the Potato virus X vector system. J. Gen. Plant Pathol. 2004, 70, 239–242. [Google Scholar] [CrossRef]
- Snippe, M.; Borst, J.W.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus Gc and N proteins interact in vivo. Virology 2007, 357, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, T.O.; Peralta, S.M.G.; Bacheller, N.; Uiterwaal, S.; Knapp, A.; Hennen, A.; Ochoa-Martinez, D.L.; Garcia-Ruiz, H. Antiviral RNA silencing suppression activity of Tomato spotted wilt virus NSs protein. Genet. Mol. Res. 2016, 15, 15028625. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, B.; Ding, Z.; Li, G.; Liu, M.; Zhu, D.; Sun, Y.; Dong, S.; Lou, Z. Distinct mechanism for the formation of the ribonucleoprotein complex of Tomato spotted wilt virus. J. Virol. 2017, 91, e00892-17. [Google Scholar] [CrossRef] [PubMed]
- Yeturu, S.; Viera, W.; Garrido, P.; Insuasti, M. First report of Tomato spotted wilt virus infecting tree tomato (Solanum Betaceum cav.) in Ecuador. J. Plant Phytopathol. 2016, 98, 691. [Google Scholar]
- Sivaprasad, Y.; Garrido, P.; Mendez, K.; Pachacama, S.; Garrido, A.; Ramos, L. First report of Tomato spotted wilt virus infecting pepper in Ecuador. J. Phytopathol. 2017, 99, 304. [Google Scholar]
- Oliver, J.E.; Whitfield, A.E. The genus tospovirus: Emerging Bunyaviruses that threaten food security. Ann. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef]
- Martinez-Ochoa, N.; Mandal, B.; Csinos, A.S. Evaluation of rhizobacteria to control tomato spotted wilt in tobacco. Phytopathology 2002, 92, S150. [Google Scholar]
- Mandal, B.; Pappu, H.R.; Csinos, A.S.; Culbreath, A.K. Response of peanut, pepper, tobacco, and tomato cultivars to two biologically distinct isolates of Tomato spotted wilt virus. Plant Dis. 2006, 90, 1150–1155. [Google Scholar] [CrossRef]
- Sun, X.H.; Gao, L.L.; Wang, S.L.; Wang, C.L.; Yang, Y.Y.; Wang, X.Y.; Zhu, X.P. First report of Tomato spotted wilt virus infecting pumpkin in China. J. Plant Phytopathol. 2016, 98, 677–697. [Google Scholar]
- Sun, M.; Jing, C.; Chu, C.; Wu, G.; Sun, X.; Xie, Y.; Liu, Y.; Qing, L. Serological detection and molecular identification of Tomato spotted wilt virus in pepper in Chongqing. Acta Hortic. Sin. 2017, 44, 487–494. [Google Scholar]
- Mo, N.; Shi, Y.; Qin, L.; Li, Y.; Liang, Y. Cloning and sequence analysis of Tomato spotted wilt virus coat protein gene in Yangling Region of Shaanxi Province. China Veg. 2019, 3, 36–40. [Google Scholar]
- Yu, M.; Yang, C.; Wang, J.; Hou, Q.; Zhang, S.; Cao, M. First report of Tomato spotted wilt virus (TSWV) isolated from nasturtium (Tropaeolum majus L.) with a serious leaf mosaic disease in China. Plant Dis. 2020, 105, 716. [Google Scholar] [CrossRef] [PubMed]
- Sevik, M.A.; Arli-Sokmen, M. Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica 2012, 40, 87–93. [Google Scholar] [CrossRef]
- Chiapello, M.; Bosco, L.; Ciuffo, M.; Ottati, S.; Salem, N.; Rosa, C.; Tavella, L.; Turina, M. Complexity and local specificity of the virome associated with tospovirus-transmitting thrips species. J. Virol. 2021. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef]
- Turina, M.; Kormelink, R.; Resende, R.O. Resistance to tospoviruses in vegetable crops: Epidemiological and molecular aspects. Annu. Rev. Phytopathol. 2016, 54, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Zhang, Q.; Yasir, M.; Li, F. Small RNA based genetic engineering for plant viral resistance: Application in crop protection. Front. Microbiol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Carbonell, P.; Alonso, A.; Grau, A.; Francisco Salinas, J.; García-Martínez, S.; José Ruiz, J. Twenty years of tomato breeding at EPSO-UMH: Transfer resistance from wild types to local landraces-from the first molecular markers to genotyping by sequencing (GBS). Diversity 2018, 10, 12. [Google Scholar] [CrossRef]
- De Oliveira, A.S.; Boiteux, L.S.; Kormelink, R.; Resende, R.O. The Sw-5 gene cluster: Tomato breeding and research toward orthotospovirus disease control. Front. Plant Sci. 2018, 9, 1055. [Google Scholar] [CrossRef]
- Chen, Y.; Dessau, M.; Rotenberg, D.; Rasmussen, D.A.; Whitfield, A.E. Entry of bunyaviruses into plants and vectors. Adv. Virus Res. 2019, 104, 65–96. [Google Scholar]
- Zhu, M.; Van Grinsven, I.L.; Kormelink, R.; Tao, X. Paving the way to tospovirus infection: Multilined interplays with plant innate immunity. Annu. Rev. Phytopathol. 2019, 57, 41–62. [Google Scholar] [CrossRef]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef]
- Roselló, S.; Díez, M.J.; Lacasa, A.; Jordá, C.; Nuez, F. Testing resistance to TSWV introgressed from Lycopersicon peruvianum by artificial transmission techniques. Euphytica 1997, 98, 93–98. [Google Scholar] [CrossRef]
- Kabaş, A.; Fidan, H.; Demirelli, M.B. Identification of new sources of resistance to resistance-breaking isolates of Tomato spotted wilt virus. Saudi J. Biol. Sci. 2021, 28, 3094–3099. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Ma, Q.; Shekasteband, R.; Stewart, K.S.; Hutton, S.F.; Scott, J.W.; Fei, Z.; Ling, K.-S. Comprehensive transcriptome analysis and functional characterization of PR-5 for its involvement in tomato Sw-7 resistance to Tomato spotted wilt tospovirus. Sci. Rep. 2019, 9, 7673. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gresa, M.P.; Lison, P.; Yenush, L.; Conejero, V.; Rodrigo, I.; Belles, J.M. Salicylic acid is involved in the basal resistance of tomato plants to citrus exocortis viroid and Tomato spotted wilt virus. PLoS ONE 2016, 11, e0166938. [Google Scholar] [CrossRef]
- Shi, Y.; Mo, N.; Qi, S.; Liang, Y. Screening of resistant germplasm of Tomato spotted wilt virus and optimization of artificial identification method. China Veg. 2020, 6, 39–43. [Google Scholar]
- Lacasa, A.; Contreras, J.; Jordá, C.; Díez, M.J.; Roselló, S.; Catalá, M.S.; Costa, J.; Nuez, F. Screening of resistant materials of Lycopersicon spp. to TSWV by means of thrips transmision. Tomato Genet. Coop. Rep. 1994, 44, 16–19. [Google Scholar]
- Mandal, B.; Csinos, A.S.; Martinez-Ochoa, N.; Pappu, H.R. A rapid and efficient inoculation method for Tomato spotted wilt tospovirus. J. Virol. Methods 2008, 149, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Roselló, S.; Soler, S.; José Díez, M.; Rambla, J.L.; Richarte, C.; Nuez, F. New sources for high resistance of tomato to the Tomato spotted wilt virus from Lycopersicon peruvianum. Plant Breed. 1999, 118, 425–429. [Google Scholar] [CrossRef]
- Canady, M.A.; Stevens, M.R.; Barineau, M.S.; Scott, J.W. Tomato spotted wilt virus (TSWV) resistance in tomato derived from Lycopersicon chilense Dun. LA 1938. Euphytica 2001, 117, 19–25. [Google Scholar] [CrossRef]
- Finlay, K.W. Inheritance of spotted wilt resistance in the tomato. II. Five genes controlling spotted wilt resistance in four tomato types. Aust. J. Biol. Sci. 1953, 6, 153–163. [Google Scholar] [CrossRef]
- Roselló, S.; José Díez, M.; Nuez, F. Genetics of Tomato spotted wilt virus resistance coming from Lycopersicon peruvianum. Eur. J. Plant Pathol. 1998, 104, 499–509. [Google Scholar] [CrossRef]
- Maluf, W.R.; Toma-Braghini, M.; Corte, R.D. Progress in breeding tomatoes for resistance to Tomato spotted wilt. Braz. J. Genet. 1991, 14, 509–525. [Google Scholar]
- Zhu, M.; Jiang, L.; Bai, B.H.; Zhao, W.Y.; Chen, X.J.; Li, J.; Liu, Y.; Chen, Z.Q.; Wang, B.T.; Wang, C.L.; et al. The intracellular immune receptor Sw-5b confers broad-spectrum resistance to Tospoviruses through recognition of a conserved 21-amino acid viral effector epitope. Plant Cell 2017, 29, 2214–2232. [Google Scholar] [CrossRef]
- Paterson, R.; Scott, S.; Gergerich, R. Resistance in two Lycopersicon species to an Arkansas isolate of Tomato spotted wilt virus. Euphytica 1989, 43, 173–178. [Google Scholar] [CrossRef]
- Giordano, L.D.; de Avila, A.C.; Charchar, J.M.; Boiteux, L.S.; Ferraz, E. ‘Viradoro’: A tospovirus-resistant processing tomato cultivar adapted to tropical environments. Hortscience 2000, 35, 1368–1370. [Google Scholar] [CrossRef]
- Brommonschenkel, S.H.; Frary, A.; Frary, A.; Tanksley, S.D. The broad-spectrum tospovirus resistance gene Sw-5 of tomato is a homolog of the root-knot nematode resistance gene Mi. Mol. Plant Microbe Interact. 2000, 13, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Spassova, M.I.; Prins, T.W.; Folkertsma, R.T.; Klein-Lankhorst, R.M.; Hille, J.; Goldbach, R.W.; Prins, M. The tomato gene Sw5 is a member of the coiled coil, nucleotide binding, leucine-rich repeat class of plant resistance genes and confers resistance to TSWV in tobacco. Mol. Breed. 2001, 7, 151–161. [Google Scholar] [CrossRef]
- Folkertsma, R.T.; Spassova, M.I.; Prins, M.; Stevens, M.R.; Hille, J.; Goldbach, R.W. Construction of a bacterial artificial chromosome (BAC) library of Lycopersicon esculentum cv. Stevens and its application to physically map the Sw-5 locus. Mol. Breed. 1999, 5, 197–207. [Google Scholar] [CrossRef]
- Rosello, S.; Ricarte, B.; Diez, M.J.; Nuez, F. Resistance to Tomato spotted wilt virus introgressed from Lycopersicon peruvianum in line UPV 1 may be allelic to Sw-5 and can be used to enhance the resistance of hybrids cultivars. Euphytica 2001, 119, 357–367. [Google Scholar] [CrossRef]
- Lima, G.S.D.A.; Brommonschenkel, S.H.; Ventura, G.M. Broad-spectrum resistance to tospovirus in accessions of Lycopersicon peruvianum and L. chilense. Summa Phytopathol. 2003, 29, 352–354. [Google Scholar]
- Boiteux, L.S.; Giordano, L.D.B. Screening Lycopersicon germplasm for resistance to a Brazilian isolate of Tomato spotted wilt virus (TSWV). Tomato Genet. Coop. Rep. 1993, 42, 13–14. [Google Scholar]
- Diez, M.; Rosello, S.; Lacasa, A.; Jorda, C.; Costa, J. Agronomic behavior of tomato cultivars and lines resistant to TSWV and influence of inoculation methods. Acta Hortic. 1995, 21, 527–532. [Google Scholar] [CrossRef]
- Holmes, F.O. Resistance to spotted wilt in tomato. Phytopathology 1948, 38, 467–473. [Google Scholar]
- Gilbert, J.C.; Tanaka, J.S. ‘Anahu’, an outstanding hybrid maker. Hawaii Farm Sci. 1971, 20, 6–7. [Google Scholar]
- Gardner, R.G.; Panthee, D.R. Tomato spotted wilt virus-resistant fresh-market tomato breeding lines: NC 58S, NC 123S, NC 127S, and NC 132S. Hortscience 2012, 47, 531–532. [Google Scholar] [CrossRef]
- Rehman, S.; Postma, W.; Tytgat, T.; Prins, P.; Qin, L.; Overmars, H.; Vossen, J.; Spiridon, L.-N.; Petrescu, A.-J.; Goverse, A.; et al. A secreted SPRY domain-containing protein (SPRYSEC) from the plant-parasitic nematode globodera rostochiensis interacts with a CC-NB-LRR protein from a susceptible tomato. Mol. Plant Microbe Interact. 2009, 22, 330–340. [Google Scholar] [CrossRef]
- Kumar, N.K.K.; Ullman, D.E.; Cho, J.J. Evaluation of Lycopersicon germ plasm for Tomato spotted wilt tospovirus resistance by mechanical and thrips transmission. Plant Dis. 1993, 77, 938–941. [Google Scholar] [CrossRef]
- Stevens, M.R.; Lamb, E.M.; Rhoads, D.D. Mapping the Sw-5 locus for Tomato spotted wilt virus resistance in tomatoes using RAPD and RFLP analyses. Theor. Appl. Genet. 1995, 90, 451–456. [Google Scholar] [CrossRef]
- Stevens, M.; Scott, S.; Gergerich, R. Inheritance of a gene for resistance to Tomato spotted wilt virus (TSWV) from Lycopersicon peruvianum Mill. Euphytica 1992, 59, 9–17. [Google Scholar] [CrossRef]
- Boiteux, L.S.; Giordano, L.D. Genetic basis of resistance against two tospovirus species in tomato (Lycopersicon-esculentum). Euphytica 1993, 71, 151–154. [Google Scholar] [CrossRef]
- Price, D.L.; Memmott, F.D.; Hollingsworth, A.; Scott, J.W.; Stevens, M.R. Identification of molecular markers linked to new Tomato spotted wilt virus resistance genes in tomato using AFLP analysis. Hortscience 2007, 42, 855. [Google Scholar]
- Dockter, K.G.; O’Neil, D.S.; Price, D.L.; Scott, J.; Stevens, M.R. Molecular mapping of the Tomato spotted wilt virus resistance gene Sw-7 in tomato. Hortscience 2009, 44, 1123. [Google Scholar]
- Scott, J.; Hutton, S.; Olson, S.; Stevens, M. Spotty Results in Our Sw-7 Tomato Spotted Wilt Virus Research. 2011 Tomato Disease Workshop Meeting Abstract. 2011. Available online: http://vegetablemdonline.ppath.cornell.edu/TDW/Presentations/11%20Scott_TDW_2011.pdf (accessed on 5 October 2021).
- Gordillo, L.F.; Stevens, M.R.; Millard, M.A.; Geary, B. Screening two Lycopersicon peruvianum collections for resistance to Tomato spotted wilt virus. Plant Dis. 2008, 92, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Brommonschenkel, S.H.; Tanksley, S.D. Map-based cloning of the tomato genomic region that spans the Sw-5 tospovirus resistance gene in tomato. Mol. Gen. Genet. 1997, 256, 121–126. [Google Scholar] [CrossRef]
- Andolfo, G.; Sanseverino, W.; Aversano, R.; Frusciante, L.; Ercolano, M.R. Genome-wide identification and analysis of candidate genes for disease resistance in tomato. Mol. Breed. 2014, 33, 227–233. [Google Scholar] [CrossRef]
- De Oliveira, A.S.; Koolhaas, I.; Silva Boiteux, L.; Caldararu, O.F.; Petrescu, A.-J.; Resende, R.O.; Kormelink, R. Cell death triggering and effector recognition by Sw-5 SD-CNL proteins from resistant and susceptible tomato isolines to Tomato spotted wilt virus. Mol. Plant Pathol. 2016, 17, 1442–1454. [Google Scholar] [CrossRef]
- Stevens, M.R. Localization and mapping of Sw-7, a tomato spotted wild virus resistance gene. In Proceedings of the 42nd Tomato Breeders Roundtable, Sacramento, CA, USA, 4–6 April 2009; Available online: http://tgc.ifas.ufl.edu/2009/Stevens%20SW7%20mapping.pdf (accessed on 5 October 2021).
- Dianese, E.C.; Fonseca, M.E.N.; Inoue-Nagata, A.K.; Resende, R.O.; Boiteux, L.S. Search in Solanum (section Lycopersicon) germplasm for sources of broad-spectrum resistance to four Tospovirus species. Euphytica 2011, 180, 307–319. [Google Scholar] [CrossRef]
- Hallwass, M.; de Oliveira, A.S.; de Campos Dianese, E.; Lohuis, D.; Boiteux, L.S.; Inoue-Nagata, A.K.; Resende, R.O.; Kormelink, R. The Tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5-containing resistant tomato lines and in Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Mol. Plant Pathol. 2014, 15, 871–880. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhu, M.; Jiang, L.; Zhao, W.Y.; Li, J.; Wu, J.Y.; Li, C.; Bai, B.H.; Lu, G.; Chen, H.Y.; et al. A multilayered regulatory mechanism for the autoinhibition and activation of a plant CC-NB-LRR resistance protein with an extra N-terminal domain. New Phytol. 2016, 212, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.D.; Pallás, V.; Sánchez-Navarro, J.A.; Kormelink, R.; Resende, R.O. The NSm proteins of phylogenetically related tospoviruses trigger Sw-5b–mediated resistance dissociated of their cell-to-cell movement function. Virus Res. 2017, 240, 25–34. [Google Scholar]
- Batuman, O.; Turini, T.A.; Oliveira, P.V.; Rojas, M.R.; Macedo, M.; Mellinger, H.C.; Adkins, S.; Gilbertson, R.L. First report of a resistance-breaking strain of Tomato spotted wilt virus infecting tomatoes with the Sw-5 tospovirus-resistance gene in California. Plant Dis. 2017, 101, 637. [Google Scholar] [CrossRef]
- Aramburu, J.; Marti, M. The occurrence in north-east Spain of a variant of tomato spotted wilt virus (TSWV) that breaks resistance in tomato (Lycopersicon esculentum) containing the Sw-5 gene. Plant Pathol. 2003, 52, 407. [Google Scholar] [CrossRef]
- Ciuffo, M.; Finetti-Sialer, M.M.; Gallitelli, D.; Turina, M. First report in Italy of a resistance-breaking strain of Tomato spotted wilt virus infecting tomato cultivars carrying the Sw5 resistance gene. Plant Pathol. 2005, 54, 564. [Google Scholar] [CrossRef]
- Aramburu, J.; Galipienso, L.; Soler, S.; Lopez, C. Characterization of Tomato spotted wilt virus isolates that overcome the Sw-5 resistance gene in tomato and fitness assays. Phytopathol. Mediterr. 2010, 49, 342–351. [Google Scholar]
- Lopez, C.; Aramburu, J.; Galipienso, L.; Soler, S.; Nuez, F.; Rubio, L. Evolutionary analysis of tomato Sw-5 resistance-breaking isolates of Tomato spotted wilt virus. J. Gen. Virol. 2011, 92, 210–215. [Google Scholar] [CrossRef]
- Price, D.L.; Memmott, F.D.; Scott, J.W.; Olson, S.M.; Stevens, M.R. Identification of molecular markers linked to a new Tomato spotted wilt virus resistance source in tomato. Tomato Genet. Coop. 2007, 57, 35–36. [Google Scholar]
- Scott, J.W.; Stevens, M.R.; Olson, S.M. An alternative source of resistance to Tomato spotted wilt virus. Tomato Genet. Coop. Rep. 2005, 55, 40–41. [Google Scholar]
- Stevens, M.R.; Price, D.L.; Memmott, F.D.; Scott, J.W.; Olson, S.M. Identification of Markers Linked to Sw-7 a New Tomato Spotted Wilt Virus Resistance Gene, Derived from S. chilense. Abstracts from the 2007 Tomato Breeders Roundtable. The Pennsylvania State University, Pennsylvania, USA. Available online: http://tgc.ifas.ufl.edu/2007/2007IndividualAbsPDf/Identification%20of%20Markers%20Linked%20to%20Sw.pdf (accessed on 8 September 2010).
- Stevens, M.R.; Scott, S.J.; Gergerich, R.C. Evaluation of seven Lycopersicon species for resistance to Tomato spotted wilt virus (TSWV). Euphytica 1994, 80, 79–84. [Google Scholar] [CrossRef]
- Stevens, M.R.; Scott, J.W.; Cho, J.J.; Geary, B.D.; Memmott, F.D. A new dominantly inherited source of TSWV resistance in tomato derived from L. chilense, which resists isolates that overcome Sw-5. Hortscience 2006, 41, 991. [Google Scholar] [CrossRef]
- Padmabhan, C.; Zheng, Y.; Shekaste-Band, R.; Stewart, K.; Scott, J.; Fei, Z.; Ling, K. Identification of defense-related genes associated with tomato Sw-7 line against Tomato spotted wilt virus in tomato through transcriptome analysis. Phytopathology 2016, 106, 161. [Google Scholar]
- El-Sappah, A.H.; Islam, M.M.; El-Awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.; Liang, Y. Tomato natural resistance genes in controlling the root-knot nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, I.R.; Maluf, W.R.; Figueira, A.R.; Menezes, C.B.; de Resende, J.T.V.; Faria, M.V.; Nogueira, D.W. Marker assisted identification of tospovirus resistant tomato genotypes in segregating progenies. Sci. Agric. 2009, 66, 298–303. [Google Scholar] [CrossRef]
- Chagué, V.; Mercier, J.C.; Guénard, M.; de Courcel, A.; Vedel, F. Identification and mapping on chromosome 9 of RAPD markers linked to Sw-5 in tomato by bulked segregant analysis. Theor. Appl. Genet. 1996, 92, 1045–1051. [Google Scholar] [CrossRef]
- Śmiech, M.; Rusinowski, Z.; Malepszy, S.; Niemirowicz-Szczytt, K. New RAPD markers of Tomato spotted wilt virus (TSWV) resistance in Lycopersicon esculentum Mill. Acta Physiol. Plant 2000, 22, 299–303. [Google Scholar] [CrossRef]
- Garland, S.; Sharman, M.; Persley, D.; McGrath, D. The development of an improved PCR-based marker system for Sw-5, an important TSWV resistance gene of tomato. Aust. J. Agric. Res. 2005, 56, 285–289. [Google Scholar] [CrossRef]
- Dianese, E.C.; de Fonseca, M.E.N.; Goldbach, R.; Kormelink, R.; Inoue-Nagata, A.K.; Resende, R.O.; Boiteux, L.S. Development of a locus-specific, co-dominant SCAR marker for assisted-selection of the Sw-5 (Tospovirus resistance) gene cluster in a wide range of tomato accessions. Mol. Breed. 2010, 25, 133–142. [Google Scholar] [CrossRef]
- Shi, A.; Vierling, R.; Grazzini, R.; Chen, P.; Caton, H.; Panthee, D. Identification of molecular markers for Sw-5 gene of Tomato spotted wilt virus resistance. Am. J. Biotechnol. Mol. Sci. 2011, 1, 2159–3698. [Google Scholar] [CrossRef]
- Panthee, D.R.; Ibrahem, R. New molecular markers associated with the Sw-5 gene conferring resistance to Tomato spotted wilt virus in tomato. J. Hortic. Sci. Biotechnol. 2013, 88, 129–134. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, B.; Bae, C.; Kang, W.-H.; Kang, B.-C.; Yeam, I.; Oh, C.-S. Development of a single-nucleotide polymorphism marker for the Sw-5b gene conferring disease resistance to Tomato spotted wilt virus in tomato. Hortic. Sci. Technol. 2015, 33, 730–736. [Google Scholar] [CrossRef]
- Devran, Z.; Kahveci, E. Development and validation of a user-friendly KASP marker for the Sw-5 locus in tomato. Australas. Plant Pathol. 2019, 48, 503–507. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Ganal, M.W.; Prince, J.P.; Devicente, M.C.; Bonierbale, M.W.; Broun, P.; Fulton, T.M.; Giovannoni, J.J.; Grandillo, S.; Martin, G.B.; et al. High-density molecular linkage maps of the tomato and potato genomes. Genetics 1992, 132, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.V.; Williams, S.; Kappagantu, M.; Mitter, N.; Pappu, H.R. Transcriptome-wide identification of host genes targeted by Tomato spotted wilt virus-derived small interfering RNAs. Virus Res. 2017, 238, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395-1–aaf6395-8. [Google Scholar] [CrossRef]
- Caplan, J.; Padmanabhan, M.; Dinesh-Kumar, S.P. Plant NB-LRR immune receptors: From recognition to transcriptional reprogramming. Cell Host Microbe 2008, 3, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant immunity: From aignaling to epigenetic control of defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef]
- Tian, H.; Wu, Z.; Chen, S.; Ao, K.; Huang, W.; Yaghmaiean, H.; Sun, T.; Xu, F.; Zhang, Y.; Wang, S.; et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature 2021, 1–7. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Scholthof, K.-B.G. Plant immune responses against viruses: How does a virus cause disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef]
- Chen, H.; Qian, X.; Chen, X.; Yang, T.; Feng, M.; Chen, J.; Cheng, R.; Hong, H.; Zheng, Y.; Mei, Y.; et al. Cytoplasmic and nuclear Sw-5b NLR act both independently and synergistically to confer full host defense against tospovirus infection. New Phytol. 2021, 231, 2262–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Z.; Wu, Q.; Cai, Y.; Zhang, Y.; Zhao, R.; Yan, J.; Qian, X.; Li, J.; Zhu, M.; et al. The Sw-5b NLR nucleotide-binding domain plays a role in oligomerization and its self-association is important for the activation of cell death signaling. J. Exp. Bot. 2021, 72, 6581–6595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, H.N.; Zhu, M.; Huang, S.; Zhang, W.H.; Dinesh-Kumar, S.P.; Tao, X.R. A plant immune receptor adopts a two-step recognition mechanism to enhance viral effector perception. Mol. Plant 2019, 12, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Zamora, O.; Schulze, S.; Azoulay-Shemer, T.; Parik, H.; Unt, J.; Brosché, M.; Schroeder, J.I.; Yarmolinsky, D.; Kollist, H. Jasmonic acid and salicylic acid play minor roles in stomatal regulation by CO2, abscisic acid, darkness, vapor pressure deficit, and ozone. Plant J. 2021, 1–44. [Google Scholar] [CrossRef]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhao, P.; Zhang, X.; Yao, X.; Sun, Y.; Fang, R.; Ye, J. The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance. PLoS Pathog. 2019, 15, e1007897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Q.; Tong, C.; Chen, H.; Miao, D.; Qian, X.; Zhao, X.; Jiang, L.; Tao, X. Characterization of the roles of SGT1/RAR1, EDS1/NDR1, NPR1, and NRC/ADR1/NRG1 in Sw-5b-mediated resistance to Tomato spotted wilt virus. Viruses 2021, 13, 1447. [Google Scholar] [CrossRef] [PubMed]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef]
- Nachappa, P.; Challacombe, J.; Margolies, D.C.; Nechols, J.R.; Whitfield, A.E.; Rotenberg, D. Tomato spotted wilt virus benefits its thrips vector by modulating metabolic and plant defense pathways in tomato. Front. Plant Sci. 2020, 11, 575564. [Google Scholar] [CrossRef]
- Huang, C. From player to pawn: Viral avirulence factors involved in plant immunity. Viruses 2021, 13, 688. [Google Scholar] [CrossRef]
- Leon-Reyes, A.; Van der Does, D.; De Lange, E.S.; Delker, C.; Wasternack, C.; Van Wees, S.C.M.; Ritsema, T.; Pieterse, C.M.J. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Plant Sci. 2010, 232, 1423–1432. [Google Scholar] [CrossRef]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant-Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef]
- Mitter, N.; Koundal, V.; Williams, S.; Pappu, H. Differential expression of Tomato spotted wilt virus-derived viral small RNAs in infected commercial and experimental host plants. PLoS ONE 2013, 8, e76276. [Google Scholar] [CrossRef] [PubMed]
- Konakalla, N.C.; Bag, S.; Deraniyagala, A.S.; Culbreath, A.K.; Pappu, H.R. Induction of plant resistance in tobacco (Nicotiana tabacum) against Tomato spotted wilt orthotospovirus through foliar application of dsRNA. Viruses 2021, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W.; Voinnet, O. Antiviral immunity directed by Small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Yang, G.-S.; Chen, W.-T.; Mao, Z.-C.; Kang, H.-X.; Chen, G.-H.; Yang, Y.-H.; Xie, B.-Y. Genome-wide identification of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene 2012, 501, 52–62. [Google Scholar] [CrossRef]
- Kim, Y.J.; Maizel, A.; Chen, X. Traffic into silence: Endomembranes and post-transcriptional RNA silencing. EMBO J. 2014, 33, 968–980. [Google Scholar] [CrossRef]
- Islam, W.; ul Islam, S.; Qasim, M.; Wang, L. Host-Pathogen interactions modulated by small RNAs. RNA Biol. 2017, 14, 891–904. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Katiyar-Agarwal, S.; Morgan, R.; Dahlbeck, D.; Borsani, O.; Villegas, A.; Zhu, J.-K.; Staskawicz, B.J.; Jin, H. A Pathogen-inducible endogenous siRNA in plant immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 18002–18007. [Google Scholar] [CrossRef]
- Olaya, C.; Fletcher, S.J.; Zhai, Y.; Peters, J.; Margaria, P.; Winter, S.; Mitter, N.; Pappu, H.R. The Tomato spotted wilt virus (TSWV) genome is differentially targeted in TSWV-infected tomato (Solanum lycopersicum) with or without Sw-5 gene. Viruses 2020, 12, 363. [Google Scholar] [CrossRef]
- Ying, X.B.; Dong, L.; Zhu, H.; Duan, C.G.; Du, Q.S.; Lv, D.Q.; Fang, Y.Y.; Garcia, J.A.; Fang, R.X.; Guo, H.S. RNA-dependent RNA polymerase 1 from Nicotiana tabacum suppresses RNA silencing and enhances viral infection in Nicotiana benthamiana. Plant Cell 2010, 22, 1358–1372. [Google Scholar] [CrossRef]
- Giner, A.; Lakatos, L.; García-Chapa, M.; López-Moya, J.J.; Burgyán, J. Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog. 2010, 6, e1000996. [Google Scholar] [CrossRef]
- Hedil, M.; Kormelink, R. Viral RNA silencing suppression: The enigma of bunyavirus NSs proteins. Viruses 2016, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, G.; Sanseverino, W.; Rombauts, S.; Van de Peer, Y.; Bradeen, J.M.; Carputo, D.; Frusciante, L.; Ercolano, M.R. Overview of tomato (Solanum lycopersicum) candidate pathogen recognition genes reveals important Solanum R locus dynamics. New Phytol. 2013, 197, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yang, J.; Sun, S.; Yang, W. Identification and analysis of resistance-like genes in the tomato genome. J. Phytopathol. 2014, 162, 137–146. [Google Scholar] [CrossRef]
- Gielen, J.J.L.; de Haan, P.; Kool, A.J.; Peters, D.; van Grinsven, M.Q.J.M.; Goldbach, R.W. Engineered resistance to Tomato spotted wilt virus, a negative-strand RNA virus. Nat. Biotechnol. 1991, 9, 1363–1367. [Google Scholar] [CrossRef]
- Prins, M.; Kikkert, M.; Ismayadi, C.; de Graauw, W.; de Haan, P.; Goldbach, R. Characterization of RNA-mediated resistance to Tomato spotted wilt virus in transgenic tobacco plants expressing NSm gene sequences. Plant Mol. Biol. 1997, 33, 235–243. [Google Scholar] [CrossRef]
- Peng, J.-C.; Chen, T.-C.; Raja, J.A.J.; Yang, C.-F.; Chien, W.-C.; Lin, C.-H.; Liu, F.-L.; Wu, H.-W.; Yeh, S.-D. Broad-spectrum transgenic resistance against distinct tospovirus species at the genus level. PLoS ONE 2014, 9, e96073. [Google Scholar] [CrossRef]
- Yazhisai, U.; Rajagopalan, P.A.; Raja, J.A.J.; Chen, T.-C.; Yeh, S.-D. Untranslatable tospoviral NSs fragment coupled with L conserved region enhances transgenic resistance against the homologous virus and a serologically unrelated tospovirus. Transgenic Res. 2015, 24, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Herrero, S.; Culbreath, A.K.; Csinos, A.S.; Pappu, H.R.; Rufty, R.C.; Daub, M.E. Nucleocapsid gene-mediated transgenic resistance provides protection against Tomato spotted wilt virus epidemics in the field. Phytopathology 2000, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Tsumuki, H. Analysis of RNA-mediated virus resistance by NSs and NSm gene sequences from Tomato spotted wilt virus. Plant Sci. 2004, 166, 771–778. [Google Scholar] [CrossRef]
- Tabein, S.; Jansen, M.; Noris, E.; Vaira, A.M.; Marian, D.; Behjatnia, S.A.A.; Accotto, G.P.; Miozzi, L. The induction of an effective dsRNA-mediated resistance against Tomato spotted wilt virus by exogenous application of double-stranded RNA largely depends on the selection of the viral RNA target region. Front. Plant Sci. 2020, 11, 533338. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.V.; Nuñez, J.K.; Doudna, J.A. Biology and applications of CRISPR systems: Harnessing nature’s toolbox for genome engineering. Cell 2016, 164, 29–44. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Andolfo, G.; Iovieno, P.; Frusciante, L.; Ercolano, M.R. Genome-editing technologies for enhancing plant disease resistance. Front. Plant Sci. 2016, 7, 1813. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Steele, J.F.C.; Segretin, M.E.; Bozkurt, T.O.; Zhou, J.; Robatzek, S.; Banfield, M.J.; Pais, M.; Kamoun, S. Tomato I2 immune receptor can be engineered to confer partial resistance to the oomycete Phytophthora infestans in addition to the fungus Fusarium oxysporum. Mol. Plant-Microbe Interact. 2015, 28, 1316–1329. [Google Scholar] [CrossRef]
- Zhang, M.; Coaker, G. Harnessing effector-triggered immunity for durable disease resistance. Phytopathology 2017, 107, 912–919. [Google Scholar] [CrossRef]
- Li, S.; Shen, L.; Hu, P.; Liu, Q.; Zhu, X.; Qian, Q.; Wang, K.; Wang, Y. Developing disease-resistant thermosensitive male sterile rice by multiplex gene editing. J. Integr. Plant Biol. 2019, 61, 1201–1205. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Q.; Yi, X.; An, H.; Zhao, Y.; Ma, S.; Zhou, G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018, 16, 1415–1423. [Google Scholar] [CrossRef]
- Dahan-Meir, T.; Filler-Hayut, S.; Melamed-Bessudo, C.; Bocobza, S.; Czosnek, H.; Aharoni, A.; Levy, A.A. Efficient in planta gene targeting in tomato using geminiviral replicons and the CRISPR/Cas9 system. Plant J. 2018, 95, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Tamborski, J.; Krasileva, K.V. Evolution of plant NLRs: From natural history to precise modifications. Ann. Rev. Virol. 2020, 71, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, S.; Li, J.; Wang, H.; Zhao, Y.; Feng, M.; Dai, J.; Wang, T.; Zhu, M.; Tao, X. Stepwise artificial evolution of an Sw-5b immune receptor extends its resistance spectrum against resistance-breaking isolates of Tomato spotted wilt virus. Plant Biotechnol. J. 2021, 1–13. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/ijms222010978
