Lipids are significant nutrients for humans and help many functional and regulatory activities in the human body, such as signal transduction, myelination, and synaptic plasticity. Lipids are also involved in the structural developments of the human body . In food, lipid content and fatty acid composition are the two critical congenital parameters to the susceptibility of food to oxidative changes. Lipid content and the fatty acid composition of fat of farm animals varies significantly depending on animal species and the diet. Lipid oxidation causes quality deterioration in food. Depending upon the reaction mechanisms and factors involved, lipid oxidation can be divided into autoxidation, photo-oxidation, and enzyme-catalyzed oxidation. Autoxidation is the most common process of lipid oxidation in foods and is divided into initiation, propagation, and termination stages.
- lipid oxidation
- primary oxidation products
- secondary oxidation products
- antioxidant capacity
1. Introduction
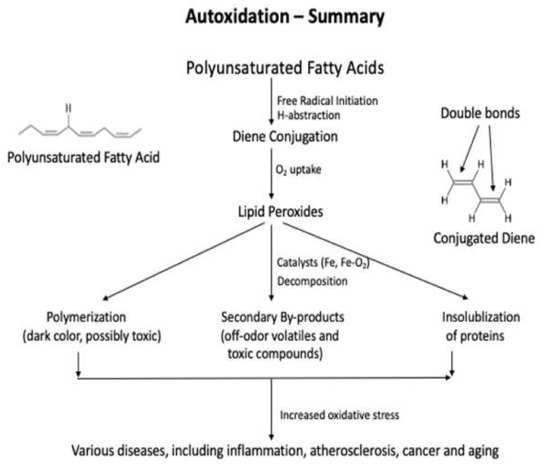
2. Methods Used to Detect the Primary Oxidation Products
2.1. Methods Used to Detect the Primary Oxidation Products
2.1.1. Peroxide Values: Iodometric and Ferric Thiocyanate Assays
The ferric irons (Fe3+) formed Equations (3) and (4) make complexes with thiocyanate, and the absorbance at 500 nm determines the amount of hydroperoxide present in the sample [9]. The final amount of Fe2+ oxidized depends on the nature of the solvent and the amount of LOOH present. However, the amount of ferrous iron oxidized depends on the amount of LOOH present in the sample when the solvent conditions are the same. The ferric thiocyanate method is simpler than the iodometric method, and the ferrous iron has lower sensitivity to oxygen than the iodide [10]. This method also has been successfully used to determine lipid oxidation levels in insect-based foods [11][12]. The method is easy, rapid, and sensitive and is responsive to mono- and polyunsaturated fatty acids (PUFA) hydroperoxides.
2.1.2. Conjugated Diene Analysis
2.2. Direct Methods to Determine the Secondary Oxidation Products
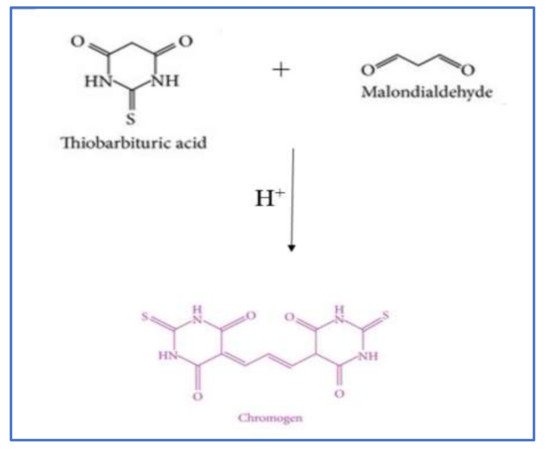
2.2.1. Thiobarbituric Acid Reactive Substances (TBARS) Method
Absorption Spectrometry
Fluorometric Spectrometry
2.2.2. Chromatographic Methods
2.3. Indirect Methods Used to Detect the Secondary Oxidation Products
2.3.1. Fluorometric Method
2.3.2. Sensory Analysis
2.4. Methods Used to Detect the Primary and Secondary Oxidation Products
| Lipid Oxidation Analysis Method | Principle of the Method | Possible Applications | Advantages of the Method | Disadvantages of the Method | References |
|---|---|---|---|---|---|
| Peroxide value (PV): Iodometric and ferric thiocyanate | Oxidation of iodide by hydroperoxides or by oxidation of Fe2+ to Fe3+. Use a spectrophotometer to obtain the final reading. |
Plant oils and liquid food products, edible insects. | Simple and cheap. Direct readings. Under anaerobic conditions, the sensitivity is high. |
Depend on the titration skills of the person. Only applicable to liquid-based products. |
[7][8][9][10][11][12] |
| Conjugated diene analysis | Isomeric hydroperoxides will make conjugated dienes with the removal of oxygen and determine 1,4-dienes produced. Measured at 233 nm. |
Suitable for PUFA-containing foods. | Gives actual values of LDL oxidation during the early stages of oxidation. Simple and cheap. |
Depend on the composition and size of the lipoproteins. Small, conjugated dienes are difficult to detect. |
[4][6][13][14] |
| TBARS assay | Detect the production of chromogen due to the reaction of MA and TBA. Read absorption at 532 nm. HPLC, GC-MS, and fluorometer are also used. |
Meat and meat-based products. Fish and fish-based products. Can be used in cured meat products, edible insects. |
Simple and fast detection. Easy to detect and low cost. Reproducible and correlate well with sensory attributes. |
MA and TBA can react with other organic compounds present in food. Absorption spectrophotometer is not suitable for detection at low levels. | [19][22][15][16][17][18][20][21][23][24][25][26] |
| Chromatography methods | By using the HPLC or GC. Determine the specific compounds produced. | All types of raw and processed foods. Oxidative stress-related diseases. | Sensitive and accurate. Identification and quantification can be made. | The cost of the equipment is high. The complexity of the method and the fact that it is time-consuming. | [19][16][23][24][32][33][34][35] |
| Fluorometric method | Use different fluorescent porphyrins to interact with MDA produced during oxidation. | Animal-based products. Can be used to determine the changes in human serum/plasma. Low moisture foods. | Fast and accurate. Non-destructive Sensitive. Image produced can be further used. Can be used to detect unstable oxidized compounds. |
High cost of the equipment used. Complexity of the method. |
[36][26][37][38][39][40][41] |
| Sensory analysis | Use trained or untrained human panelist to determine the level of oxidation through sensory attributes, such as odor, taste, and acceptability. | All animals and plant-based foods, cereals. | Gives the overall quality of the food. Direct interpretation. Can be used for liquid, semi-solid, and solid foods. |
Depends on the individual participants and time variations. Depends on the region. Reproducibility difficult. Ethical clearance is needed. |
[42][43][44][45][46] |
| p-Anisidine test | Determine the level of anisidine produced from the secondary aldehydes produced. | Oil and oil-based products. | Simple, less technical knowledge needed. | Problems in omega-3-rich oils that contain intense colors or containing specific flavorings. | [47][48][49] |
| Total oxidation index (TOTOX) | Determine the total oxidizied products. | Oil and oil-based products. | Simple calculation. | Similar problem associated with p-anisidine test. | [47][48][49] |
This entry is adapted from the peer-reviewed paper 10.3390/antiox10101587
References
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163.
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Rad. Res. 2010, 44, 1098–1124.
- Ayala, A.; Munoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nominal. Oxid. Med. Cell Longev. 2014, 360438.
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285.
- Barthel, G.; Grosch, W. Peroxide value determination—Comparison of some methods. JAOCS 1974, 51, 540–544.
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Method Enzymol. 1978, 52, 302–310.
- Pryor, W.A.; Castle, L. Chemical methods for the detection of lipid hydroperoxides. Method Enzymol. 1984, 5, 293–299.
- Labeque, R.; Marnett, L.J. 10-Hydroperoxy-8,12-octadecadienoic acid: A diagnostic probe of alkoxyl radical generation in metal-hydroperoxide reactions. J. Am. Chem. Soc. 1987, 109, 2828–2829.
- Wagner, C.D.; Clever, H.L.; Peters, E.D. Evaluation of the ferrous thiocyanate colorimetric method. Anal. Chem. 1947, 19, 980–982.
- Mihalević, B.; Katušin-Ražem, B.; Ražem, D. The revaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radic. Biol. Med. 1996, 21, 53–63.
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible insect processing pathways and implementation of emerging technologies. J. Insects Food Feed. 2021, 7, 877–890.
- Tenyang, N.; Tiencheu, B.; Mamat, A.; Mawamba, L.A.; Ponka, R. Effect of cooking methods on the nutritive value and lipid oxidation of two Cricket species consumed in Cameroon. Eur. J. Nutr. Food Saf. 2021, 13, 11–23.
- Recknagel, R.O.; Glende, E.A., Jr. Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol. 1984, 105, 331–337.
- Corongiu, F.P.; Milia, A. An improved and simple method for determining diene conjugation in autoxidized polyunsaturated fatty acids. Chem. Biol. Interact. 1983, 44, 289–297.
- Jo, C.; Ahn, D.U. Fluorometric analysis of 2-thiobarbituric acid reactive substances in turkey. Poultry Sci. 1998, 77, 475–480.
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94.
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 9412767.
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198.
- Kunyaboon, S.; Thumanu, K.; Park, J.W.; Khongla, C.; Yongsawatdigul, J. Evaluation of lipid oxidation, volatile compounds and vibrational spectroscopy of silver carp (Hypophthalmichthys molitrix) during ice storage as related to the quality of its washed mince. Foods 2021, 10, 495.
- Salih, A.; Smith, D.; Price, J.; Dawson, L. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poultry Sci. 1987, 66, 1483–1488.
- Ahn, D.U.; Sell, J.L.; Jeffery, M.; Jo, C.; Chen, X.; Wu, C.; Lee, J.I. Dietary vitamin E affects lipid oxidation and total volatiles of irradiated raw turkey meat. J. Food Sci. 1997, 62, 954–959.
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48.
- Ahn, D.U.; Olson, D.G.; Jo, C.; Chen, X.; Wu, C.; Lee, J.I. Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production and color in raw pork patties. Meat Sci. 1998, 49, 27–39.
- Schaich, K.M. Analysis of lipid and protein oxidation in fats, oils and foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats, 1st ed.; Hu, M., Jaconsen, C., Eds.; AOCS Press: Cambridge, MA, USA, 2016; pp. 1–131.
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611.
- Aubourg, S.P. Recent advances in assessment of marine lipid oxidation by using fluorescence. J. Am. Chem. Soc. 1999, 76, 409–419.
- Bernheim, F.; Bernheim, M.L.C.; Wilbur, K.M. The reaction between thiobarbituric acid and the oxidation products of certain lipids. J. Biol. Chem. 1948, 174, 257–264.
- Kosugi, H.; Kikugawa, K. Potential thiobarbiuturic acid-reactive substances in peroxidized lipids. Free Radic. Biol. Med. 1989, 7, 205–208.
- Pérez-Palacios, T.; Estévez, M. Chapter 13—Analysis of lipids and lipid oxidation products. In Meat Quality Analysis—Advanced Evaluation Methods, Techniques, and Technologies; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 217–239.
- Yagi, K. Lipid Peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351.
- Kakuda, Y.; Stanlry, D.W.; van deVoort, F.R. Determination of TBA number by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1981, 58, 773–775.
- Kato, S.; Shimizu, N.; Hanzawa, Y.; Otoki, Y.; Ito, J.; Kimura, F.; Takekoshi, S.; Sakaino, M.; Sano, T.; Eitsuka, T.; et al. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography-tandem mass spectrometry. NPJ Sci. Food 2018, 2.
- Steinhorst-Slikkerveer, L.; Louter, A.; Janssen, H.G.; Bauer-Plank, C. Analysis of nonvolatile lipid oxidation products in vegetable oils by normal-phase high-performance liquid chromatography with mass spectrometric detection. J. Am. Oil Chem. Soc. 2000, 8, 837–845.
- Ahn, D.U.; Olson, D.G.; Jo, C.; Love, J.; Jin, S.K. Volatile production and lipid oxidation of irradiated coocked sausages with different packaging during storage. J. Food Sci. 1999, 64, 226–229.
- Lazaridi, E.; Janssen, H.-G.; Vincken, J.-P.; Pirok, B.; Hennbelle, M. A comprehensive two-dimensional liquid chromatography method for the simultaneous separation of lipid species and their oxidation products. J. Chromatogr. A 2021, 1644, 462106.
- Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 22, 1988.
- Pikul, J.; Leszczynski, D.E.; Bechtel, P.J.; Kummerow, F.A. Effects of frozen storage and cooking on lipid oxidation in chicken meat. J. Food Sci. 1984, 49, 838–843.
- Tappel, A.L. Vitamin E and free radical peroxidation of lipids. Ann. N. Y. Acad. Sci. 1972, 203, 12–28.
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture foods: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482.
- Hassoun, A.; Karoui, R. Front-face fluorescence spectroscopy coupled with chemometric tools for monitoring fish freshness stored under different refrigerated conditions. Food Control 2015, 54, 240–249.
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. Assessment of lipid oxidation in Atlantic mackerel (Scomber scombrus) subjected to different antioxidant and sous-vide cooking treatments by conventional and fluorescence microscopy methods. Food Control 2019, 104, 1–8.
- Fruhwirth, G.O.; Wenzl, T.; El-Toukhy, R.; Wagner, F.S.; Hermetter, A. Fluorescence screening of antioxidant capacity in pumpkin seed oils and other natural oils. Eur. J. Lipid Sci. Technol. 2003, 105, 266–274.
- Mihafu, F.D.; Issa, J.Y.; Kamiyango, M.W. Implication of sensory evaluation and quality assessment in food product development: A review. Curr. Res. Nutr. Food Sci. 2020, 8, 690–702.
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating volatile lipid oxidation compounds with consumer sensory data in dairy-based powders during storage. Antioxidants 2020, 9, 338.
- Fan, Y.; Odabasi, A.; Sims, C.; Gao, Z.; Sarnoski, P. Utilization of descriptive sensory analysis and volatile analysis to determine quality indicators of aquacultured whiteleg shrimp (Litopanaeus vannemei) during Refrigerated Storage. J. Aquatic Food Prod. Technol. 2020, 29, 722–735.
- Paradiso, V.M.; Summo, C.; Pasqualone, A.; Caponio, F. Evaluation of different natural antioxidants as affectivg volatile lipid oxidation products related to off-flavours in corn flakes. Food Chem. 2009, 113, 543–549.
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf-Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157.
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781.
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 3–4.
