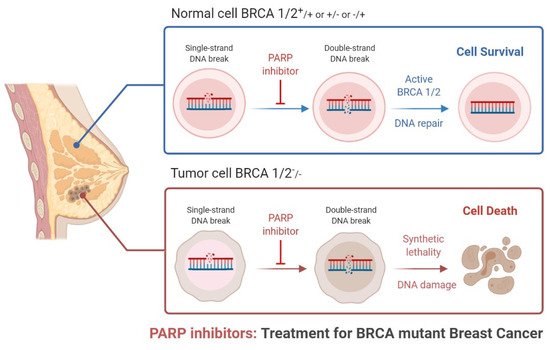
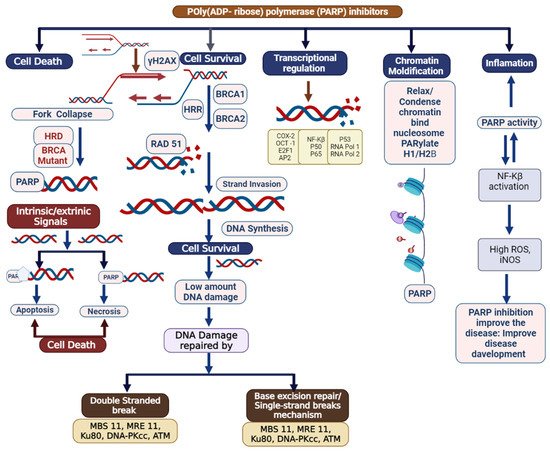
Triple-negative breast cancer is a combative cancer type with a highly inflated histological grade that leads to poor theragnostic value. Gene, protein, and receptor-specific targets have shown effective clinical outcomes in patients with TNBC. Cells are frequently exposed to DNA-damaging agents. DNA damage is repaired by multiple pathways; accumulations of mutations occur due to damage to one or more pathways and lead to alterations in normal cellular mechanisms, which lead to development of tumors. Advances in target-specific cancer therapies have shown significant momentum; most treatment options cause off-target toxicity and side effects on healthy tissues. PARP (poly(ADP-ribose) polymerase) is a major protein and is involved in DNA repair pathways, base excision repair (BER) mechanisms, homologous recombination (HR), and nonhomologous end-joining (NEJ) deficiency-based repair mechanisms. DNA damage repair deficits cause an increased risk of tumor formation. Inhibitors of PARP favorably kill cancer cells in BRCA-mutations. For a few years, PARPi has shown promising activity as a chemotherapeutic agent in BRCA1- or BRCA2-associated breast cancers, and in combination with chemotherapy in triple-negative breast cancer.
- breast cancer
- PARP (poly(ADP-ribose) polymerase)
- TNBC
- therapeutic target
- DNA damage repair
- signaling pathway
1. Introduction
2. Clinical Applications of PARP Inhibitors in TNBC
| Compound Name | Compound Structure | Efficacy | IC50 |
|---|---|---|---|
| Nicotinamide | 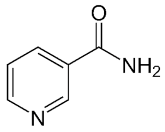 |
PARP inhibitor and by-product of the PARP reaction; many pharmacological actions other than that of inhibiting PARP | 210 μM |
| 3-aminobenzamide | 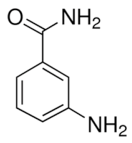 |
Benzamides are free radical scavengers, among other pharmacological actions |
33 μM |
| PD128763 | 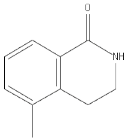 |
Cytoprotective agent, chemosensitizer, and radiosensitizer; adverse effect of compound causes hypothermia | 420 nM |
| DPQ | 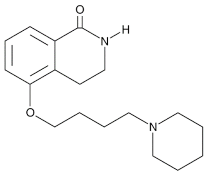 |
A commonly used Warner–Lambert PARP inhibitor compound based on an isoquinoline core |
1 μM |
| NU1025 | 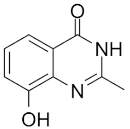 |
Potentiators of anticancer agent cytotoxicity |
400 nM |
| 4-ANI | 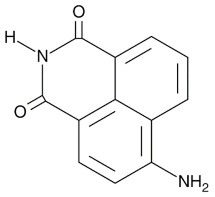 |
PARP in DNA repair and cell death | 180 nM |
| ISO | 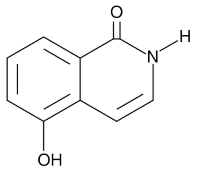 |
PARP in DNA repair and cell death | 390 nM |
| Olaparib (Lynparza) |
 |
Use in a BRCA1-positive patient with metastatic triple-negative breast cancer, without the initial use of platinum-based chemotherapy, showed significant rapid near-resolution of large liver metastasis while patient experienced gout-like symptoms | 1 nM |
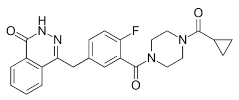
| Niraparib (Zejula) | 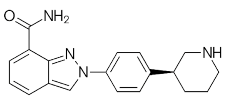 |
Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer | 4 nM |
| Talazoparib (Talzenna) |
 |
Ferm line BRCA-mutant, HER2-negative locally advanced or metastatic breast cancer | 0.6 nM |
| Veliparib (ABT-888) | 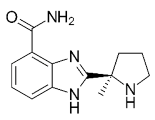 |
Received orphan drug status for lung cancer |
2 nM |
| INO-1001 | 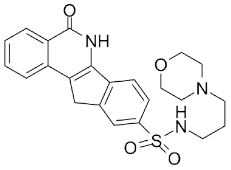 |
Potent enhancer of radiation sensitivity and enhances radiation-induced cell killing by interfering with DNA repair mechanisms, resulting in necrotic cell death | 105 nM |
| E7449 | 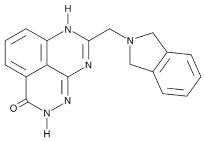 |
Antitumor activity of E7449; a novel PARP 1/2 and tankyrase 1/2 inhibitor | 1 nM |
| CEP-8983 | 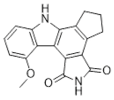 |
Increases the sensitivity of chemoresistant tumor cells to temozolomide | 20 nM |
| Pamiparib (BGB-290) |
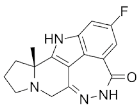 |
Pamiparib has potent PARP trapping, the capability to penetrate the brain, and can be used for the research of various cancers including solid tumors | 0.9 nM |
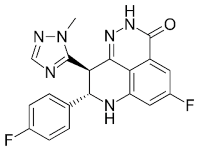
| Fluzoparib (SHR-3162) |
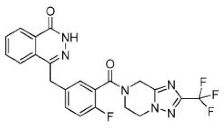 |
Inhibitor of poly-adenosine diphosphate(ADP)ribose polymerase (PARP) 1/2 being developed for the treatment of BRCA1/2-mutant solid tumors. | 1.5 nM |
| Name of the Molecules | Tmax (h) | t (h) | AUC (lgh/ mL) | Cmax (lg/mL) | CL/F (L/h) | Vz/F | References |
|---|---|---|---|---|---|---|---|
| Olaparib capsule formulation 300 mg | 1.49 (0.57–3.05) |
13.02 (8.23) | 55.20 (67.4) | 8.05 (24.3) | 6.36 (3.47) | 112.1 (59.84) | [18] |
| Olaparib tablet formulation 300 mg single dose (fasted) | 1.50 (0.50–5.85) |
12.2 (5.31) |
43.6 (54.3) [AUCt] 43.0 (55.2) [AUC] |
7.00 (35.0) | 7.95 (4.23) | 146 (142) | [19] |
| Olaparib tablet formulation 300 mg single dose (fed) | 4.00 (1.00–12.0) |
12.2 (5.31) |
46.0 (56.6) [AUCt] 45.4 (57.1) [AUC] |
5.48 (40.5) | 7.55 (3.99) | 127 (107) | [19] |
| Veliparib monotherapy 40 mg (10 mg, fasting) | 1.2 ± 0.8 | 5.9 ± 1.3 | 2.23 ± 0.82 [AUCt] 2.43 ± 1.07 [AUC] |
0.36 ± 0.13 | 19.0 ± 7.36 | NA | [20][21] |
| Veliparib monotherapy 40 mg (10 mg, fed) | 1.2 ± 0.7 | 5.8 ± 1.2 | 2.45 ± 0.93 [AUCt] 2.65 ± 1.17 [AUCt] |
0.37 ± 0.12 | 17.3 ± 6.41 | NA | |
| Veliparib monotherapy 40 mg (40 mg, fasting) | 1.3 ± 0.9 | 5.8 ± 1.3 | 2.24 ± 0.98 [AUCt] 2.45 ± 1.24 [AUCt] |
0.34 ± 0.12 | 19.5 ± 7.66 | NA | |
| Veliparib monotherapy 40 mg (40 mg, fed) | 2.5 ± 1.1 | 5.8 ± 1.4 | 2.14 ± 0.80 [AUCt] 2.35 ± 1.06 [AUCt] |
0.28 ± 0.09 | 19.7 ± 7.51 | NA | |
| Veliparib metabolite M8 | 2.4 (3.5–9.8) | – | 0.3–1.9 [AUCint] |
0.011 (0.007–0.014) |
NA | NA | [20][21] |
| Niraparib 300 mg/day | 3.1 (2.0–6.1) | a | 14.117 (AUC24)b |
1.921 | NA | NA | [12] |
| Niraparib metabolite: unlabeled M1 plasma | 9.02 | 78.4 | 41.2 (AUCt) | 476 | NA | NA | [15] |
| Name of Drug | Types of Inhibitors | Prior Treatment | Type of Population | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| AZD1775 in patent with TNBC LYNPARZATM |
PARP Inhibitor, patent with TNBC |
Olaparib in combination with AZD6738 mutated (ATM) | Inhibitor of Ataxia-Telangiectasia and WEE1 inhibitor |
Phase II | NCT03330847 |
| AZD1775 in patent with TNBC LYNPARZATM |
PARP Inhibitor, patent with TNBC |
Olaparib with radiation therapy, after chemotherapy |
Inhibitor of ataxia-telangiectasia |
Phase I | NCT03109080 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with atezolizumab | Inhibitor of PD-L1 |
Phase II | NCT02849496 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Oolaparib with paclitaxel and carboplatin |
Inhibitor of germline BRCA mutated |
Phase II/III | NCT03150576, NCT02789332 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with AZD2171 orally |
Inhibitor of VEGFR tyrosine kinase | Phase I/II | NCT01116648 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with PI3K inhibitor, BKM120 | Inhibitor of BKM120 | Phase I | NCT01623349 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with onalespib | Inhibitor of heat shock protein 90 | Phase I | NCT02898207 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with AZD2014 |
mTORC1/2 or Oral AKT inhibitor |
Phase I/II | NCT02208375 |
| PARP1/2 inhibitor Veliparib |
Patent with TNBC | Veliparib in combination with cyclophosphamide |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Phase II and failed in phase III trials |
NCT01306032 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of tyrosine kinase, HER2, and BRCA |
Veliparib in combination with carboplatin |
Patients with TNBC | Completed phase I study | NCT01251874 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, BRCA, and tyrosine kinase |
Veliparib with vinorelbine |
Patients with TNBC | Completed phase I | NCT01281150 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with cisplatin | Patients with TNBC | Completed phase I | NCT01104259 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with pegylation | Patients with TNBC | Completed phase I | NCT01145430 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with pegylation | Patients with TNBC | Completed phase I | NCT01145430 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with lapatinib | Patients with TNBC | Phase I | NCT02158507 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib in combination with irinotecan HCl | Patients with TNBC | Phase I I | NCT00576654 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with cisplatin |
Patients with TNBC | Phase II | NCT02595905 |
| AZD2281 and Ku-0059436 PARP1/2 inhibitor (Selective) |
PARP inhibitor; BRCA Mutated |
Olaparib alone, or in combination with durvalumab MEDI4736 against PD-L1 |
HER2-negative treated mTNBC |
Phase-II | NCT00679783 NCT03544125 NCT02484404 NCT03167619 NCT02681562 NCT02484404 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib plus carboplatin | Patients with TNBC | Phase III | NCT02032277 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Combination with gemcitabine and carboplatin |
Patients with TNBC | Phase II | NCT00813956 NCT01045304 NCT01130259 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Combination of iniparib with paclitaxel for TNBC compared to paclitaxel alone |
Patients with TNBC | Competed phase II | NCT01204125 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Iniparib with irinotecan | Patients with TNBC | Phase II trial | NCT01173497 |
| Niraparib | ≥1 anti-HER2 treatment; PARP inhibitor |
Niraparib plus trastuzumab IV |
Metastatic HER2+ breast cancer |
Phase Ib/II (recruiting) | NCT03368729 |
| Niraparib | PARP inhibitor | One anthracycline and/or taxane in the (neo-) adjuvant or Niraparib |
Advanced/metastatic BRCA1- like |
Phase-II, Active, not recruiting | NCT02826512 |
| Niraparib | PARP inhibitor | ≥1 line of therapy Niraparib plus everolimus |
Patients with TNBC | Phase I Recruiting | NCT03154281 |
| Niraparib | Germline BRCA mutation-positive (PARP inhibitors) |
≤2 prior cytotoxic regimens and Niraparib versus physician‘s choice |
Advanced or metastatic breast cancer |
Phase III Active, not yet recruiting |
NCT01905592 (BRAVO) |
| Niraparib | Metastatic TNBC inhibitors (PARP inhibitors) |
≤2 lines of cytotoxic therapy, Niraparib plus pembrolizumab |
Advanced or metastatic TNBC |
Phase I/II Active, not yet recruiting |
NCT02657889 (KEYNOTE-162) |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
≤2 lines of cytotoxic Chemotherapy, Carboplatin, and paclitaxel with or without veliparib |
Locally advanced unresectable BRCA associated |
Phase III Recruiting | NCT02163694 |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
Veliparib with temozolomide versus veliparib with carboplatin and paclitaxel versus placebo with carboplatin and paclitaxel ≤2 lines of cytotoxic chemotherapy |
Metastatic TNBC |
Randomized phase II, Ongoing |
NCT01506609 |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
Veliparib versus atezolizumab versus veliparib plus atezolizumab |
Stage III–IV TNBC | Randomized phase II Ongoing |
NCT02849496 |
| veliparib | Metastatic TNBC inhibitors PARP inhibitors) |
Cisplatin and placebo versus cisplatin and veliparib ≤1 line of cytotoxic chemotherapy for metastatic disease |
Metastatic TNBC and/or BRCA mutation-associated breast cancer |
Phase II Active, not recruiting | NCT02595905 |
| veliparib | Metastatic TNBC inhibitors PARP inhibitors) |
Temozolomide and veliparib ≥1 chemotherapy regimen |
Metastatic TNBC and/or BRCA mutation-associated breast cancer |
Phase II, Active, not recruiting | NCT01009788 |
| Talazoparib | Neoadjuvant therapy | None | Primary breast cancer ≥1 cm with a deleterious BRCA mutation |
Phase II, Active, not recruiting | NCT02282345 |
| Talazoparib | Advanced TNBC and HR deficiency and advanced HER2-negative breast cancer or other solid tumors with a mutation in HR pathway genes |
≥1 line of therapy | Talazoparib | Phase II, Recruiting | NCT02401347 |
| Talazoparib | Metastatic TNBC inhibitors PARP inhibitors |
Platinum-containing regimen with disease progression > 8 weeks |
Metastatic breast cancer with BRCA mutation |
Phase II Terminated (Primary Analysis and study completed Not stopped | NCT02034916 (ABRAZO) |
| Talazoparib | Metastatic TNBC inhibitors PARP inhibitors |
≤3 chemotherapy-inclusive regimens Talazoparib versus physician‘s choice |
Locally advanced and/or metastatic breast cancer with germline BRCA mutations |
Phase III Completed | NCT01945775 (EMBRACA) |
| Rucaparib | Metastatic TNBC inhibitors PARP inhibitors |
≤5 prior chemotherapy Rucaparib regimens in the last 5 years |
Patients presenting with metastatic breast cancer (MBC) | Phase II, Completed | NCT00664781 |
| Rucaparib | Metastatic TNBC inhibitors PARP inhibitors |
≥1 line of chemotherapy, Rucaparib | Patients with a BRCAness genomic signature |
Phase II Completed | NCT02505048 (RUBY) |
| Rucaparib | Stage I–III patients with TNBC or inhibitors PARP inhibitors |
Neoadjuvant chemotherapy Cisplatin with rucaparib | ER/PR+, HER2- negative breast cancer with known BRCA1/2 mutations |
Phase II Completed | NCT01074970 |
| Rucaparib | TNBC inhibitors | ≥3 prior chemotherapy regimens, Rucaparib |
Patients with advanced solid tumors with evidence of germline |
Phase I/II Active, not recruiting |
NCT01482715 |
| Rucaparib | TNBC inhibitors | ≤5 prior chemotherapy regimens in the last 5 years, Rucaparib |
Patients with MBC carriers of a BRCA1/2 | Phase II Completed | NCT00664781 |
| Rucaparib | TNBC inhibitors | ≥1 line of chemotherapy Rucaparib | Patients with a BRCAness genomic signature |
Phase II Completed | NCT02505048 (RUBY) |
| Rucaparib | TNBC inhibitors | Neoadjuvant chemotherapy Cisplatin with rucaparib |
Advanced solid tumors with evidence of germline or somatic BRCA mutation |
Completed | NCT01074970 |
| Rucaparib | TNBC inhibitors | ≥3 prior chemotherapy regimens |
Advanced solid tumors with evidence of germline or somatic BRCA mutation |
Phase I/II Active, not recruiting |
NCT01482715 |
2.1. Olaparib
2.2. Iniparib (BSI-201)
2.3. Niraparib
2.4. Veliparib (ABT-888)
2.5. Talazoparib (BMN-673)
2.6. Rucaparib
2.7. Checkpoint Inhibitors
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9111512
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Rummel, S.K.; Lovejoy, L.; Shriver, C.D.; Ellsworth, R.E. Contribution of germline mutations in cancer predisposition genes to tumor etiology in young women diagnosed with invasive breast cancer. Breast Cancer Res. Treat. 2017, 164, 593–601.
- Singh, D.D.; Yadav, D.K. TNBC: Potential Targeting of Multiple Receptors for a Therapeutic Breakthrough, Nanomedicine, and Immunotherapy. Biomedicines 2021, 9, 876.
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández Hernández, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078.
- Lee, K.-L.; Chen, G.; Chen, T.-Y.; Kuo, Y.-C.; Su, Y.-K. Effects of Cancer Stem Cells in Triple-Negative Breast Cancer and Brain Metastasis: Challenges and Solutions. Cancers 2020, 12, 2122.
- Lee, K.-L.; Kuo, Y.-C.; Ho, Y.-S.; Huang, Y.-H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334.
- Nederlof, I.; Horlings, H.; Curtis, C.; Kok, M. A High-Dimensional Window into the Micro-Environment of Triple Negative Breast Cancer. Cancers 2021, 13, 316.
- Keung, M.Y.T.; Wu, Y.; Vadgama, J.V. PARP Inhibitors as a Therapeutic Agent for Homologous Recombination Deficiency in Breast Cancers. J. Clin. Med. 2019, 8, 435.
- Schettini, F.; Giudici, F.; Bernocchi, O.; Sirico, M.; Corona, S.P.; Giuliano, M.; Locci, M.; Paris, I.; Scambia, G.; De Placido, S.; et al. Poly (ADP-ribose) polymerase inhibitors in solid tumours: Systematic review and meta-analysis. Eur. J. Cancer 2021, 149, 134–152.
- Gourley, C.; Balmaña, J.; Ledermann, J.A.; Serra, V.; Dent, R.; Loibl, S.; Pujade-Lauraine, E.; Boulton, S.J. Moving From Poly (ADP-Ribose) Polymerase Inhibition to Targeting DNA Repair and DNA Damage Response in Cancer Therapy. J. Clin. Oncol. 2019, 37, 2257–2269.
- van Beek, L.; McClay, É.; Patel, S.; Schimpl, M.; Spagnolo, L.; de Oliveira, T.M. PARP Power: A Structural Perspective on PARP1, PARP2, and PARP3 in DNA Damage Repair and Nucleosome Remodelling. Int. J. Mol. Sci. 2021, 22, 5112.
- Langelier, M.-F.; Planck, J.L.; Roy, S.; Pascal, J.M. Structural Basis for DNA Damage-Dependent Poly(ADP-Ribosyl)Ation by Human PARP-1. Science 2012, 336, 728–732.
- Pascal, J.M. The Comings and Goings of PARP-1 in Response to DNA Damage. DNA Repair 2018, 71, 177–182.
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair 2019, 81, 102651.
- Van Andel, L.; Zhang, Z.; Lu, S.; Kansra, V.; Agarwal, S.; Hughes, L.; Tibben, M.M.; Gebretensae, A.; Lucas, L.; Hillebrand, M.J.X.; et al. Human mass balance study and metabolite profiling of 14Cniraparib, a novel poly(ADP-Ribose) polymerase (PARP)-1 and PARP-2 inhibitor, in patients with advanced cancer. Investig. New Drugs 2017, 35, 751–765.
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N. Engl. J. Med. 2009, 361, 123–134.
- Virtanen, V.; Paunu, K.; Ahlskog, J.K.; Varnai, R.; Sipeky, C.; Sundvall, M. PARP Inhibitors in Prostate Cancer—The Preclinical Rationale and Current Clinical Development. Genes 2019, 10, 565.
- Dirix, L.; Swaisland, H.; Verheul, H.M.; Rottey, S.; Leunen, K.; Jerusalem, G.; Rolfo, C.; Nielsen, D.; Molife, L.R.; Kristeleit, R.; et al. Effect of itraconazole and rifampin on the pharmacokinetics of olaparib in patients with advanced solid tumors: Results of two phase I open-label studies. Clin. Ther. 2016, 38, 2286–2299.
- Plummer, R.; Swaisland, H.; Leunen, K.; Van Herpen, C.M.L.; Jerusalem, G.; De Greve, J.; Lolkema, M.P.; Soetekouw, P.; Mau-Sørensen, M.; Nielsen, D.; et al. Olaparib tablet formulation: Effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 2015, 76, 723–729.
- Mostafa, N.M.; Chiu, Y.L.; Rosen, L.S.; Bessudo, A.; Kovacs, X.; Giranda, V.L. A phase 1 study to evaluate effect of food on veliparib pharmacokinetics and relative bioavailability in subjects with solid tumors. Cancer Chemother. Pharmacol. 2014, 74, 583–591.
- Tuli, R.; Shiao, S.L.; Nissen, N.; Tighiouart, M.; Kim, S.; Osipov, A.; Bryant, M.; Ristow, L.; Placencio-Hickok, V.; Hoffman, D.; et al. A Phase 1 Study of Veliparib, a PARP-1/2 Inhibitor, with Gemcitabine and Radiotherapy in Locally Advanced Pancreatic Cancer. EBioMedicine 2019, 40, 375–381.
- Andreidesz, K.; Koszegi, B.; Kovacs, D.; Bagone Vantus, V.; Gallyas, F.; Kovacs, K. Effect of Oxaliplatin, Olaparib and LY294002 in Combination on Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 2056.
- Sari, M.; Saip, P. Myelodysplastic Syndrome after Olaparib Treatment in Heavily Pretreated Ovarian Carcinoma. Am. J. Ther. 2019, 26, e632–e633.
- Murai, J.; Huang, S.-Y.N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Pommier, Y. Stereospecific PARP Trapping by BMN 673 and Comparison with Olaparib and Rucaparib. Mol. Cancer Ther. 2014, 13, 433–443.
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533.
- Nicolas, E.; Bertucci, F.; Sabatier, R.; Gonçalves, A. Targeting BRCA Deficiency in Breast Cancer: What are the Clinical Evidences and the Next Perspectives? Cancers 2018, 10, 506.
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Flechon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986.
- Robson, M.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566.
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, E510.
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141.
- Nitecki, R.; Gockley, A.A.; Floyd, J.L.; Coleman, R.L.; Melamed, A.; Rauh-Hain, J.A. The incidence of myelodysplastic syndrome in patients receiving poly-ADP ribose polymerase inhibitors for treatment of solid tumors: A meta-analysis. J. Clin. Oncol. 2020, 38, 3641.
- Hossain, F.; Majumder, S.; David, J.; Miele, L. Precision Medicine and Triple-Negative Breast Cancer: Current Landscape and Future Directions. Cancers 2021, 13, 3739.
- Bergin, A.R.T.; Loi, S. Triple-negative breast cancer: Recent treatment advances. F1000Research 2019, 8, 1342.
- Pierce, A.; McGowan, P.M.; Cotter, M.; Mullooly, M.; O’Donovan, N.; Rani, S.; O’Driscoll, L.; Crown, J.; Duffy, M.J. Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines. Cancer Biol. Ther. 2013, 14, 537–545.
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 2016, 22, 3764–3773.
- Diéras, V.; Bonnefoi, H.; Alba, E.; Awada, A.; Coudert, B.; Pivot, X.; Gligorov, J.; Jager, A.; Zambelli, S.; Lindeman, G.J.; et al. Iniparib administered weekly or twice-weekly in combination with gemcitabine/carboplatin in patients with metastatic triple-negative breast cancer: A phase II randomized open-label study with pharmacokinetics. Breast Cancer Res. Treat. 2019, 177, 383–393.
- Mateo, J.; Ong, M.; Tan, D.S.; Gonzalez, M.A.; de Bono, J.S. Appraising iniparib, the PARP inhibitor that never was—What must we learn? Nat. Rev. Clin. Oncol. 2013, 10, 688–696.
- O’Shaughnessy, J.; Schwartzberg, L.; Danso, M.A.; Miller, K.D.; Rugo, H.S.; Neubauer, M.; Robert, N.; Hellerstedt, B.; Saleh, M.; Richards, P.; et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2014, 32, 3840–3847.
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402.
- Sandhu, S.K.; Schelman, W.R.; Wilding, G.; Moreno, V.; Baird, R.D.; Miranda, S.; Hylands, L.; Riisnaes, R.; Forster, M.; Omlin, A.; et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2013, 14, 882–892.
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140.
- Appleman, L.J.; Beumer, J.H.; Jiang, Y.; Lin, Y.; Ding, F.; Puhalla, S.; Swartz, L.; Owonikoko, T.K.; Donald Harvey, R.; Stoller, R.; et al. Phase 1 study of veliparib (ABT-888), a poly (ADP-ribose) polymerase inhibitor, with carboplatin and paclitaxel in advanced solid malignancies. Cancer Chemother. Pharmacol. 2019, 84, 1289–1301.
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509.
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282.
- Han, H.S.; Diéras, V.; Robson, M.; Palácová, M.; Marcom, P.K.; Jager, A.; Bondarenko, I.; Citrin, D.; Campone, M.; Telli, M.L.; et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: Randomized phase II study. Ann. Oncol. 2018, 29, 154–161.
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629.
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535.
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients with Operable Breast Cancer with a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394.
- Miller, K.; Tong, Y.; Jones, D.R.; Walsh, T.; Danso, M.A.; Ma, C.X.; Silverman, P.; King, M.-C.; Badve, S.S.; Perkins, S.M. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: Final efficacy results of Hoosier Oncology Group BRE09-146. J. Clin. Oncol. 2015, 33, 1082.
- Drew, Y.; Ledermann, J.; Hall, G.; Rea, D.; Glasspool, R.; Highley, M.; Jayson, G.; Sludden, J.; Murray, J.; Jamieson, D.; et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br. J. Cancer 2016, 114, 723–730.
- Durmus, S.; Sparidans, R.W.; van Esch, A.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) restrict oral availability and brain accumulation of the PARP inhibitor rucaparib (AG-014699). Pharm. Res. 2015, 32, 37–46.
- Simmons, A.D.; Nguyen, M.; Pintus, E. Polyclonal BRCA2 mutations following carboplatin treatment confer resistance to the PARP inhibitor rucaparib in a patient with mCRPC: A case report. BMC Cancer 2020, 20, 215.
- FDA. Grants Accelerated Approval to Rucaparib for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistantprostate (accessed on 15 July 2021).
