The techniques currently used in 3D-assisted acetabular fracture surgery are 3D printing and visual surgical planning, 3D printing and pre-contouring of implants, and custom-made patient-specific implants. Three-dimensional-assisted surgery compared to conventional surgery reduces operation time, intraoperative blood loss, intraoperative fluoroscopy usage, and complication rate. Evidence for the improvement of postoperative fracture reduction and physical functioning is limited, because of heterogeneity and varying qualities of the studies.
- acetabular fracture
- 3D
- three-dimensional
- 3D print
- surgical planning
- systematic review
1. Introduction
Acetabular fractures are fractures involving the hip socket, which might have major impacts on the patient’s mobility, social activities, and the ability to work. These severe injuries usually occur due to high-energy trauma mechanisms (i.e., car accidents) in young patients [1]. In addition, acetabular fractures are increasingly caused by low-energy trauma mechanisms (i.e., fall at ground level) in frail elderly [1]. Adequate fracture reduction and fixation is crucial to minimise the risks on progressive posttraumatic arthritis of the hip socket and the subsequent need for revision surgery to a total hip arthroplasty [2]. Acetabular fractures are complex fractures, due to the three-dimensional (3D) geometry of the pelvis and displacement of fracture fragments in multiple directions. Insight into fracture patterns can be challenging using only two-dimensional (2D) images [3]. In the past decade, 3D technology has increasingly been used in acetabular fracture treatment. Three-dimensional printing is useful for classifying acetabular fractures and for teaching purposes [4][5][6][7]. For instance, 3D printed models may improve the quality of surgical trainees’ preoperative understanding of the spatial complexity of fractures [8]. In addition, a randomised controlled trial showed that using a 3D interactive software system for teaching acetabular fracture classification improved the classification accuracy [7]. Moreover, the use of 3D printed fracture models has improved fracture classification in comparison with 2D/3D CT images, due to enhanced tactile feedback of the complex geometry [5][6]. This may result in a shorter time needed to classify the acetabular fractures and a higher interobserver agreement as compared to the evaluation of these fractures using 2D CT images [4].
Over the past few years, the number of publications on the applications of 3D-assisted surgery in acetabular fracture treatment is rapidly increasing [9]. It encompasses a spectrum of modalities, including 3D visualisation, 3D printing, and patient-specific surgical guides or implants. Preoperative planning of the fracture reduction and pre-contouring of implants using 3D printed models has been reported in acetabular fracture surgery in case series [10][11][12][13][14][15]. For example, Hu et al. [11] created virtual 3D models of fractured acetabula based on CT images and virtually reduced the fracture fragments, in order to gain more insight into fracture patterns and treatment strategies. Moreover, the uninjured hemipelvis can be mirrored virtually and 3D printed [13][15]. This printed hemipelvis can be used as a template for the pre-contouring of implants prior to surgery [13][15]. In addition, the use of 3D printed drilling guides and patient-specific osteosynthesis plates have been described [16][17][18][19]. For instance, 3D printed drilling guides have been designed to fit temporarily on top of an implant in order to aim the drill bit and screw trajectories in the predetermined directions [19]. In addition, patient-specific implants, with or without drilling guides, have been designed based on virtual 3D models [16][17]. The application of patient-specific osteosynthesis plates provides the possibility to execute the preoperative plan and attain the predetermined osteosynthesis plate and screw positions [16]. However, comparative studies or reviews on the added clinical value of 3D-assisted acetabular fracture surgery compared to conventional surgery (i.e., defined as using only radiographs and 2D CT images in preoperative planning) are only sparingly available. Next to the surgeons’ understanding of these technologies, patients cannot be informed properly about the potential benefits of these innovations. In addition, insurance companies take evidence-based decisions on the implementation of these technologies.
2. Research Methods
The Preferred Reporting Items for Systematic Reviews (PRISMA) [20] were used. The review protocol has been registered in the PROSPERO International prospective register of systematic reviews under registration number CRD42021225274.
On 1 March 2021, the PubMed and Embase libraries were searched for articles published between 1 January 2010 and 28 February 2021. Together with a medical librarian, the search string was generated ( Table 1 ).
| Database | Search String |
|---|---|
| PubMed | (3D[tiab] OR three dimension*[tiab] OR 3 dimension*[tiab] OR ‘Printing, Three-Dimensional’ [Mesh] OR ‘Imaging, Three-Dimensional’ [Mesh]) AND (acetabul*[tiab] OR ‘Acetabulum’ [Mesh]) AND (fractur*[tiab] OR ‘Fractures, Bone’ [Mesh]) AND ‘2010/01/01’ [PDat]: ‘3000/12/31’ [PDat] |
| Embase | (‘three dimensional imaging’/exp OR ‘three dimensional printing’/exp OR ‘3 d’:ti,ab OR ‘3 dimension*’:ti,ab OR ‘three dimension*’:ti,ab) AND (‘acetabulum’/exp OR acetabul*:ti,ab) AND (‘fracture’/exp OR fractur*:ti,ab) AND [embase]/lim AND [2010,2011,2012,2013,2014,2015,2016,2017,2018,2019,2020,2021]/py |
Studies that were eligible for inclusion were randomised controlled trials, cohort studies, case-control studies, cross-sectional studies, and case series on the treatment of acetabular fractures in humans by using 3D technology. Exclusion criteria were reviews; letters to the editor or conference abstracts; cadaveric studies; case reports (N < 10); paediatric studies (age < 18 years); studies in other languages than English, German, French, or Dutch; studies on fracture classification, measurements or education; studies on intraoperative imaging or surgical navigation; and biomechanical studies. Articles were imported into Rayyan QCRI, a web-based sorting tool for systematic literature reviews [21]. Next, two reviewers (AM, FIJ) independently screened the articles for eligibility based on the titles and abstracts using the Rayyan QCRI tool. The same reviewers independently screened all remaining articles by full text. Finally, the references of the included articles were screened for additional relevant manuscripts.
The guidelines of the McMaster University Occupational Therapy Evidence-Based Practice Research Group were used to assess the methodological quality and risk of bias [22]. The McMaster critical appraisal consists of components considering the study purpose, background literature, study design, sample size, randomisation, outcome measures, study intervention, study results, conclusions, and implications. Scores were given with ‘yes = 1 point’, ‘no = 0 points’, and ‘not applicable (NA)’. The total score reflects the methodological quality with a maximum score of 16 for RCTs, 12 for case series, and 14 for other designs. The definitive score is presented as a percentage that varies from 0 to 100%, with a higher score indicating a higher methodological quality. Scores of <50% are considered poor-quality studies, scores of 50–74% are considered moderate-quality studies, scores of 75–90% are considered good-quality studies, and scores of >90% are considered excellent-quality studies. The data extraction and quality check were independently conducted (AM, FIJ) using the McMaster Critical Review Form. Disagreements were resolved in a consensus meeting.
The primary outcome of this systematic review was the surgical outcome in terms of operation time, intraoperative blood loss, intraoperative fluoroscopy usage, complications, and fracture reduction. Complications were defined as nerve injury, vascular injury, infection, thrombosis/embolism, heterotopic ossification, osteoarthritis, avascular necrosis of the femoral head, and implant failure. The quality of acetabular fracture reduction was defined by the greatest residual gap or step-off at the acetabulum on the plain radiographs or on a postoperative CT scan in any of the views [23][24]. The residual displacement was graded according to Matta’s criteria as anatomic (0 to 1 mm gap and/or step-off), imperfect (2 to 3 mm), or poor (>3 mm) [24]. An adequate reduction was defined as the Matta category anatomical and satisfactory or a postoperative displacement of ≤2 mm, and a poor reduction was defined as the Matta category poor or a postoperative displacement of >2 mm. Secondary outcome was physical functioning, assessed with the Patient-Reported Outcome Measures (PROMs) or clinician-reported outcome measures. Functional outcome was graded according to the definitions of the Modified Merle d’Aubigné (Excellent 18, Good 15–17, Fair 13–14, Poor < 13) and the Harris Hip score (Excellent 90–100, Good 80–90, Fair 70–80, Poor <70) [25][26][27][28].
The weighted mean with a standard deviation of all applicable studies was calculated, using SPSS (version 23, IBM, Chicago, IL, USA), when more than two studies reported the outcome variable. For comparative studies, the differences in continuous outcome measures were calculated by using the inverse variance weighting method and presented as the weighted mean difference (WMD) with the 95% confidence interval (CI), using Review Manager (version 5.4.1, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). For dichotomous variables, the odds ratio with the 95% CI was calculated using the Mantel–Haenszel method in Review Manager. A p -value of <0.05 was considered to indicate statistical significance. Authors were contacted to retrieve additional data, such as not reported means or their standard deviations, but retrieving additional data was unsuccessful.
3. Findings
In total, 482 studies were found. After removal of duplicates, 357 studies were screened on title and abstract. After title and abstract screening, 28 articles were included for full-text screening. Nine of these full-text articles were excluded due to the following reasons: foreign language article on 3D printing and pre-contouring the implant (N = 1); case reports (N = 2); descriptive study ( N = 1); biomechanical study (N = 1); conference abstract (N = 1); outcome measurements unclear (N = 3). In total, 19 studies met the inclusion criteria for this systematic review (Figure 1) [29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47]. The included studies enrolled a total of 753 patients (median sample size 27; range 10–146). Three-dimensional-assisted surgery was used in 478 of all the patients ( Figure 2 ). In 400 patients, a 3D print and plate pre-contouring of the implant was used (14 studies); in 69 patients, a patient-specific implant was used (three studies); and in 9 patients, only 3D printing for pre- and intraoperative fracture visualisation was used (one study). Conventional surgery, defined as preoperative planning based on radiographs and 2DCT images (axial, sagittal, and coronal views), was used in 275 patients. The study characteristics are presented in Table 2 .
| Study | Year | Country | Design | N | Period | Outcome Measurements | 3D Technology |
|---|---|---|---|---|---|---|---|
| Ansari et al. [30] | 2020 | India | Case control | 27 | August 2017–July 2018 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications, FU: Harris hip score | 3D printing and plate pre-contouring |
| Chen et al. [31] | 2019 | China | Case control | 52 | January 2013–January 2017 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications, FU: modified Merle d’Aubigné | 3D printing and plate pre-contouring; virtual plating |
| Downey et al. [40] | 2020 | Ireland | Prospective cohort | 18 | October 2017–May 2018 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications: infection | 3D printing |
| Hsu et al. [41] | 2019 | China | Case control | 29 | March 2014–February 2018 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications |
3D printing and plate pre-contouring |
| Huang et al. [38] | 2020 | China | Randomised Controlled Trial | 40 | September 2013–September 2017 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications, FU: Harris hip score | 3D printing and plate pre-contouring |
| IJpma et al. [39] | 2021 | Netherlands | Prospective case series | 10 | January 2017–December 2018 | Postoperative fracture reduction, complications, FU: Short Musculoskeletal Function Assessment | Patient-specific implants |
| Li et al. [42] | 2019 | Taiwan | Case control | 16 | September 2013–August 2017 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications | 3D printing and plate pre-contouring |
| Maini et al. [43] | 2018 | India | Randomised Controlled Trial | 21 | June 2012–December 2014 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications | 3D printing and plate pre-contouring |
| Maini et al. [44] | 2018 | India | Randomised Controlled Trial | 25 | October 2014–March 2016 | Operation time, intraoperative blood loss, postoperative fracture reduction | 3D printing of virtually pre-contoured plates as template for plate pre-contouring |
| Öztürk et al. [45] | 2020 | Turkey | Case control | 18 | January 2017–June 2018 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications | 3D printing and plate pre-contouring |
| Wan et al. [46] | 2019 | China | Case control | 96 | January 2016–June 2017 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications, FU: Harris hip score | 3D printing and plate pre-contouring |
| Wang et al. [47] | 2020 | China | Case control | 50 | January 2016–June 2017 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications | Patient-specific implants |
| Wang et al. [32] | 2020 | China | Case control | 88 | February 2013–February 2016 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications, FU: Merle d’Aubigne | 3D printing and plate pre-contouring |
| Weidert et al. [33] | 2020 | Germany | Retrospective case series | 12 | NS | Operation time, intraoperative blood loss, FU: (modified) Harris hip score, Merle d’Aubigne | 3D printing and plate pre-contouring |
| Wu et al. [34] | 2020 | China | Case control | 43 | May 2014–January 2018 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications, FU: modified Merle d’Aubigne | Patient-specific implants |
| Xu et al. [29] | 2014 | China | Prospective case series | 24 | January 2008–August 2011 | Operation time, intraoperative blood loss, postoperative fracture reduction, FU: Merle d’Aubigne, complications | Patient-specific implants |
| Yu et al. [35] | 2020 | China | Case control | 146 | June 2011–December 2017 | Operation time, intraoperative blood loss, intraoperative fluoroscopy usage, postoperative fracture reduction, complications, FU: Harris hip score | 3D printing and plate pre-contouring |
| Zeng et al. [36] | 2016 | China | Prospective case series | 10 | June 2013–February 2015 | Postoperative fracture reduction, complications | 3D printing and plate pre-contouring |
| Zou et al. [37] | 2020 | China | Retrospective case series | 33 | June 2017–December 2018 | Operation time, intraoperative blood loss, postoperative fracture reduction, complications, FU: modified Merle d’Aubigne | 3D printing and plate pre-contouring |
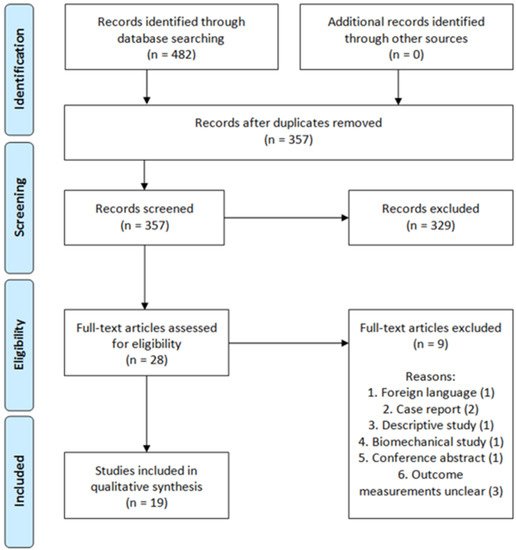
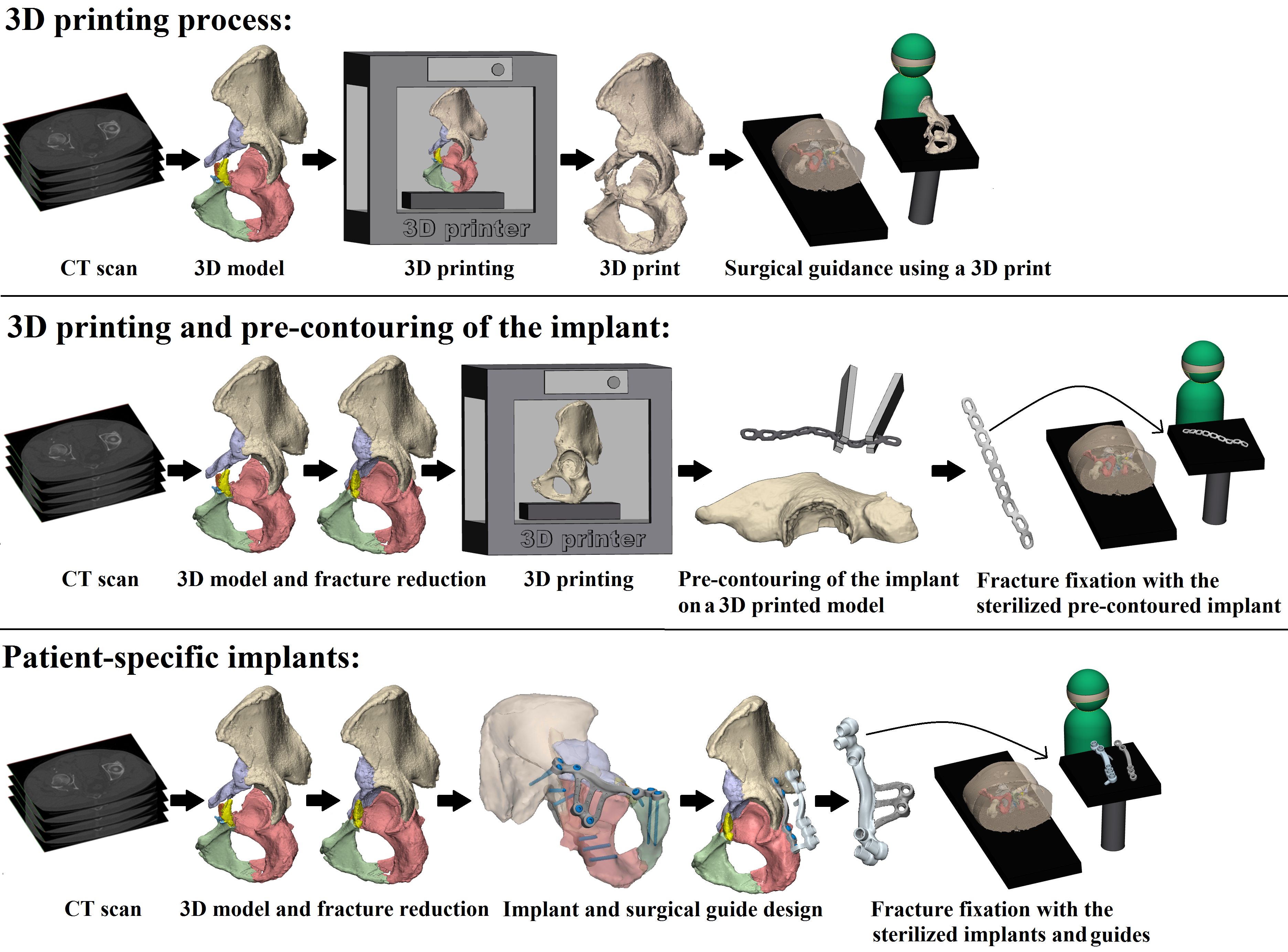 Figure 2. Three-dimensional-assisted surgery. Three-dimensional-assisted surgery encompasses a spectrum of modalities, including 3D visualisation, 3D printing, and patient-specific surgical guides or implants. The steps required for 3D printing, 3D printing and pre-contouring of the implant, or the manufacturing of patient-specific implants are illustrated. In the 3D printing process (top row) a virtual 3D model is created from a CT scan, e.g., using Mimics Medical software in which a threshold for bone tissue is selected based on the Hounsfield Units of the CT scan. The 3D models are split into the separate fragments, indicated by the different colours. This virtual model can be 3D printed and used for preoperative planning and surgical guidance. For 3D printing and pre-contouring of the implant (middle row), a virtual 3D model is created from a CT scan. Then, the contralateral healthy hemipelvis is mirrored, e.g., using 3-matic Medical software, and it is used as a template for the virtual fracture reduction. The fracture fragments are virtually reduced to their original anatomical position. The mirrored or virtually reduced hemipelvis can be 3D printed and this 3D print is used for pre-contouring of the implant. One study performed virtual plating and printed the contour of a plate, which was then used for pre-contouring the implant [44]. Next, the pre-contoured implant is sterilised and used for intraoperative fracture fixation. Finally, patient-specific implants (bottom row) are designed, based on the virtual 3D model from the CT scan. Either the mirrored contralateral pelvis or the fracture reduction can be used as a model for the implants. The screw directions and positions are predetermined and then the implant is designed based on the shape of the pelvis of the individual patient and based on the fracture type. The implant is accompanied by a surgical guide, to ensure that the screws are positioned and directed as planned. The implants and surgical guides are sterilised and used for intraoperative fracture fixation within four days.
Figure 2. Three-dimensional-assisted surgery. Three-dimensional-assisted surgery encompasses a spectrum of modalities, including 3D visualisation, 3D printing, and patient-specific surgical guides or implants. The steps required for 3D printing, 3D printing and pre-contouring of the implant, or the manufacturing of patient-specific implants are illustrated. In the 3D printing process (top row) a virtual 3D model is created from a CT scan, e.g., using Mimics Medical software in which a threshold for bone tissue is selected based on the Hounsfield Units of the CT scan. The 3D models are split into the separate fragments, indicated by the different colours. This virtual model can be 3D printed and used for preoperative planning and surgical guidance. For 3D printing and pre-contouring of the implant (middle row), a virtual 3D model is created from a CT scan. Then, the contralateral healthy hemipelvis is mirrored, e.g., using 3-matic Medical software, and it is used as a template for the virtual fracture reduction. The fracture fragments are virtually reduced to their original anatomical position. The mirrored or virtually reduced hemipelvis can be 3D printed and this 3D print is used for pre-contouring of the implant. One study performed virtual plating and printed the contour of a plate, which was then used for pre-contouring the implant [44]. Next, the pre-contoured implant is sterilised and used for intraoperative fracture fixation. Finally, patient-specific implants (bottom row) are designed, based on the virtual 3D model from the CT scan. Either the mirrored contralateral pelvis or the fracture reduction can be used as a model for the implants. The screw directions and positions are predetermined and then the implant is designed based on the shape of the pelvis of the individual patient and based on the fracture type. The implant is accompanied by a surgical guide, to ensure that the screws are positioned and directed as planned. The implants and surgical guides are sterilised and used for intraoperative fracture fixation within four days.
Three randomised controlled trials [38][43][44], one prospective cohort study [40], ten case control studies [30][31][32][34][35][41][42][45][46][47], and five case series [29][33][36][37] were included. The methodological quality of the papers varied from low ( Table 3 ) to good ( Table 4 ). The median and interquartile range (IQR) McMaster score was 69% (IQR 64–86) for all studies together and for the prospective and retrospective studies separately.
| Categories | Zou, 2020 | Weidert, 2020 | Zeng, 2016 | Öztürk, 2020 | Wan, 2019 | Xu, 2014 | Maini, 2018 1 | Li, 2019 | Wang, 2020 2 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Study purpose | |||||||||
| Was the study question clearly stated? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| 2. Literature review | |||||||||
| Was relevant background literature reviewed? | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 3. Study design | CR | CR | CR | CC | CC | CR | RCT | CC | CC |
| 4. Sample | |||||||||
| Was the sample described in detail? | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Was the sample justified? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Were the groups randomised? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Was randomising appropriate done? | NA | NA | NA | NA | NA | NA | 1 | NA | NA |
| 5. Outcomes | |||||||||
| Were the outcome measures reliable? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Were the outcome measures valid? | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| 6. Intervention | |||||||||
| Intervention was described in detail? | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Contamination was avoided? | NA | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 |
| Cointervention was avoided? | NA | NA | NA | 1 | 1 | 0 | 1 | 1 | 1 |
| 7. Results | |||||||||
| Results were reported in terms of statistical significance? | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Were the analysis method/s appropriate? | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Clinical importance was reported? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Drop-outs were reported? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8. Conclusion | |||||||||
| Conclusions were appropriate given study methods and results? | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Total | 3/12 | 5/12 | 5/12 | 8/14 | 8/14 | 7/12 | 11/16 | 10/14 | 10/14 |
| % | 25 | 42 | 42 | 57 | 57 | 58 | 69 | 71 | 71 |
Yes = 1 point, no = 0 points, CC = Case Control study, CS = Cohort study, RCT = Randomized Controlled Trial, CR = Case Series, N/A = Not applicable
1: Maini et al. (2018) - Evaluation of accuracy of virtual surgical planning for patient-specific pre-contoured plate in acetabular fracture fixation
2: Wang et al. (2020) - The effect of new preoperative preparation method compared to conventional method in complex acetabular fractures: minimum 2-year follow-up
Table 4. Quality assessment part two.
|
Categories |
Huang, 2020 |
Maini, 20183 |
Wu, 2020 |
IJpma, 2021 |
Ansari, 2020 |
Chen, 2019 |
Downey, 2020 |
Hsu, 2019 |
Wang, 20204 |
Yu, 2020 |
|
1. Study purpose |
|
|
|
|
|
|
|
|
|
|
|
Was the study question clearly stated? |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
2. Literature review |
|
|
|
|
|
|
|
|
|
|
|
Was relevant background literature reviewed? |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
0 |
|
3. Study design |
RCT |
RCT |
CC |
CS |
CC |
CC |
CS |
CC |
CC |
CC |
|
4. Sample |
|
|
|
|
|
|
|
|
|
|
|
Was the sample described in detail? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Was the sample justified? |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Were the groups randomized? |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Was randomizing appropriate done? |
0 |
1 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
|
5. Outcomes |
|
|
|
|
|
|
|
|
|
|
|
Were the outcome measures reliable? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Were the outcome measures valid? |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
6. Intervention |
|
|
|
|
|
|
|
|
|
|
|
Intervention was described in detail? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Contamination was avoided? |
1 |
1 |
1 |
NA |
1 |
1 |
1 |
1 |
1 |
1 |
|
Cointervention was avoided? |
1 |
1 |
1 |
NA |
1 |
1 |
1 |
1 |
1 |
1 |
|
7. Results |
|
|
|
|
|
|
|
|
|
|
|
Results were reported in terms of statistical significance? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Were the analysis method/s appropriate? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Clinical importance was reported? |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Drop-outs were reported? |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
8. Conclusion |
|
|
|
|
|
|
|
|
|
|
|
Conclusions were appropriate given study methods and results? |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Total |
12/16 |
12/16 |
11/14 |
10/12 |
12/14 |
12/14 |
12/14 |
12/14 |
12/14 |
12/14 |
|
% |
75 |
75 |
79 |
83 |
86 |
86 |
86 |
86 |
86 |
86 |
Yes = 1 point, no = 0 points, CC = Case Control study, CS = Cohort study, RCT = Randomized Controlled Trial, CR = Case Series, N/A = Not applicable
3: Maini et al. (2018) - Three-dimensional printing and patient-specific pre-contoured plate: future of acetabulum fracture fixation?
4: Wang et al. (2020) - Three-dimensional printing of patient-specific plates for the treatment of acetabular fractures involving quadrilateral plate disruption
The weighted mean operation time in the 3D-assisted group and in the conventional group was 162.5 ± 79.0 min versus 296.4 ± 56.0 min. Additionally, the weighted mean blood loss of all studies was 697.9 ± 235.7 mL versus 1097.2 ± 415.5 mL. Nine out of fourteen comparative studies reported a significantly shorter operation time and less blood loss when 3D-assisted surgery was performed [30][31][32][34][38][41][45][46][47]. The operation time was 43 min shorter for the 3D-assisted group compared to the conventional group, but the heterogeneity was high (Figure 3). There was 243 mL less blood loss in the 3D-assisted group compared to the conventional group, but the heterogeneity was high (Figure 4).
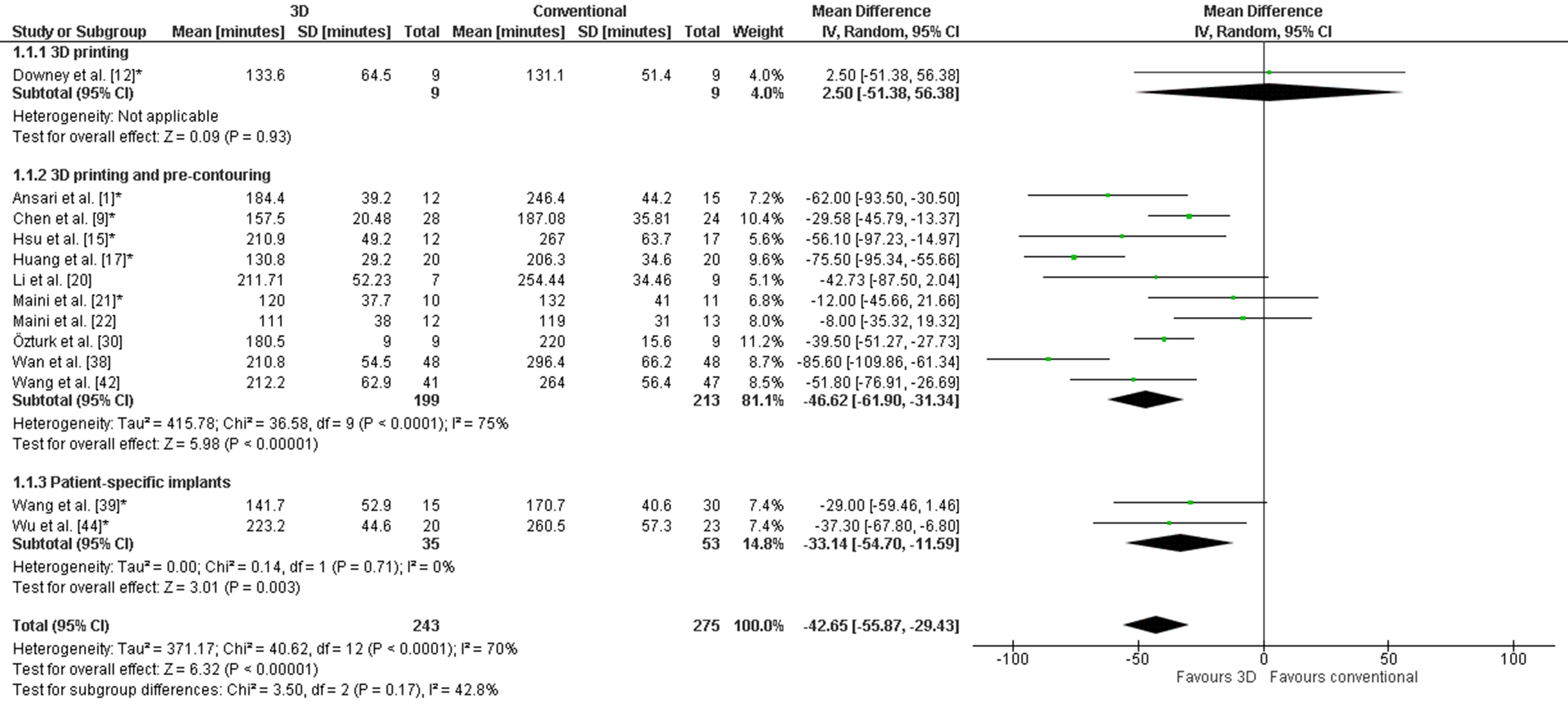 Figure 3. Forest plot of operation time. *: Good-quality study.
Figure 3. Forest plot of operation time. *: Good-quality study.
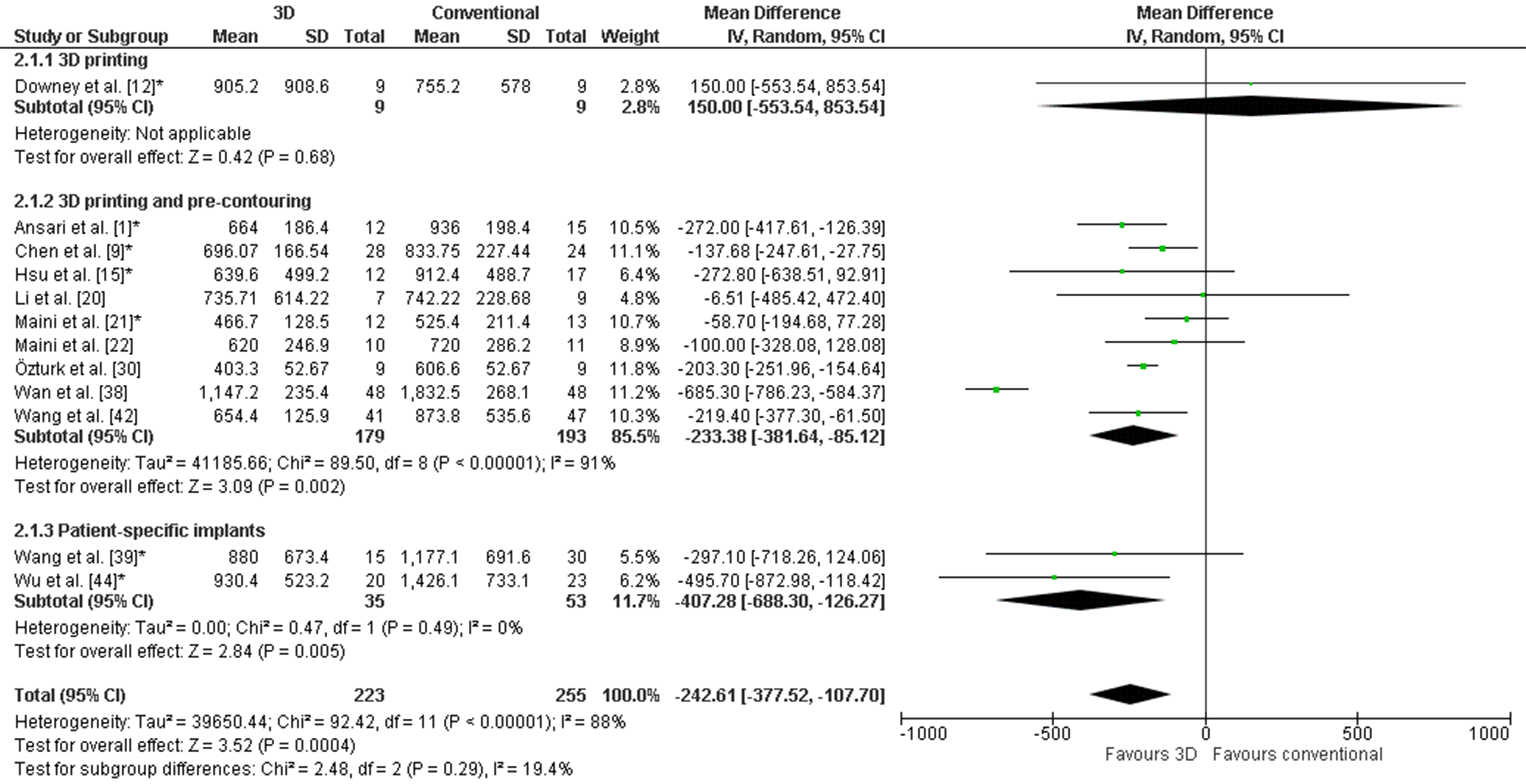 Figure 4. Forest plot of blood loss. *: Good-quality study.
Figure 4. Forest plot of blood loss. *: Good-quality study.
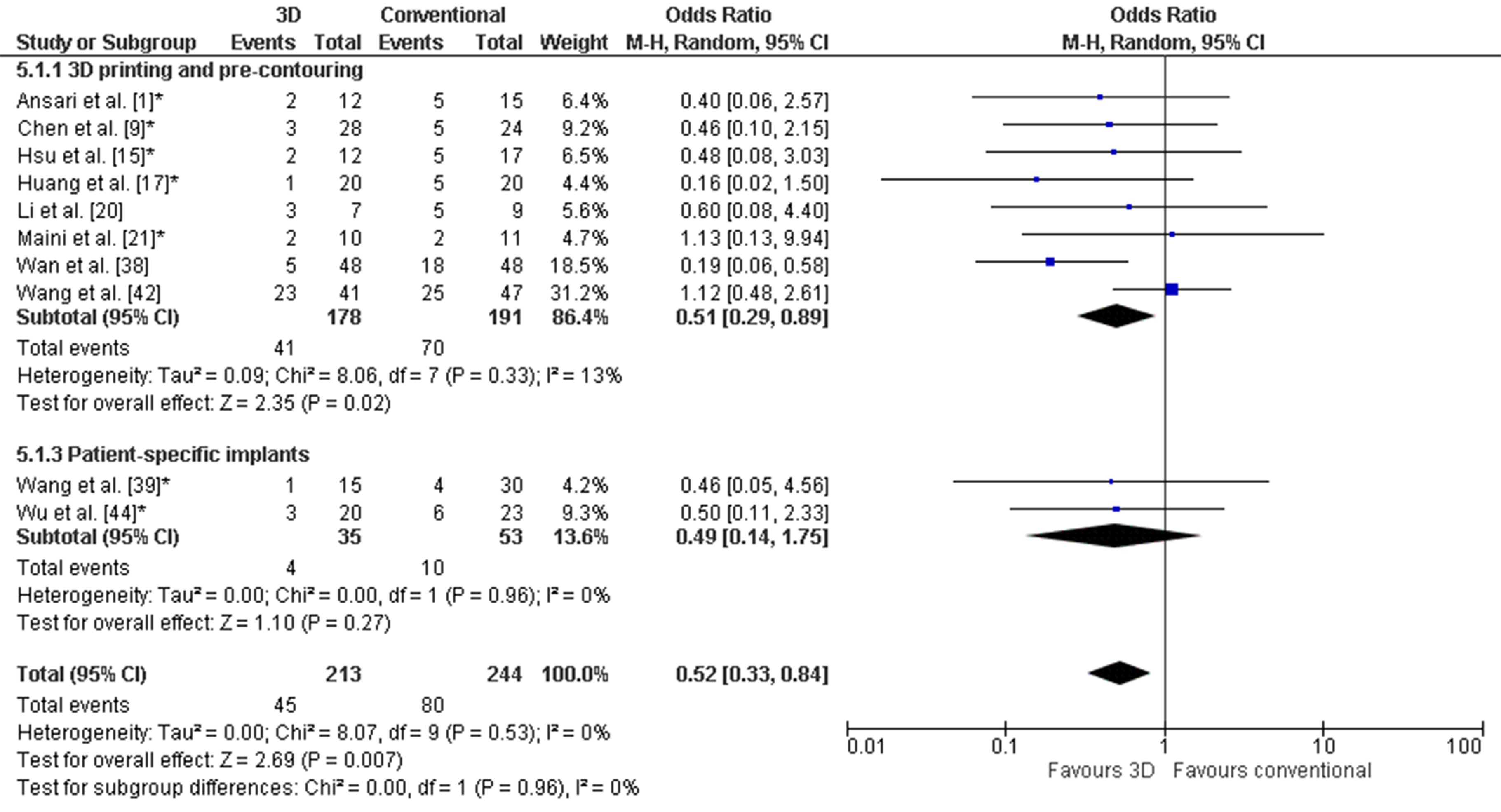 Figure 5. Forest plot of the complications. *: Good-quality study.
Figure 5. Forest plot of the complications. *: Good-quality study.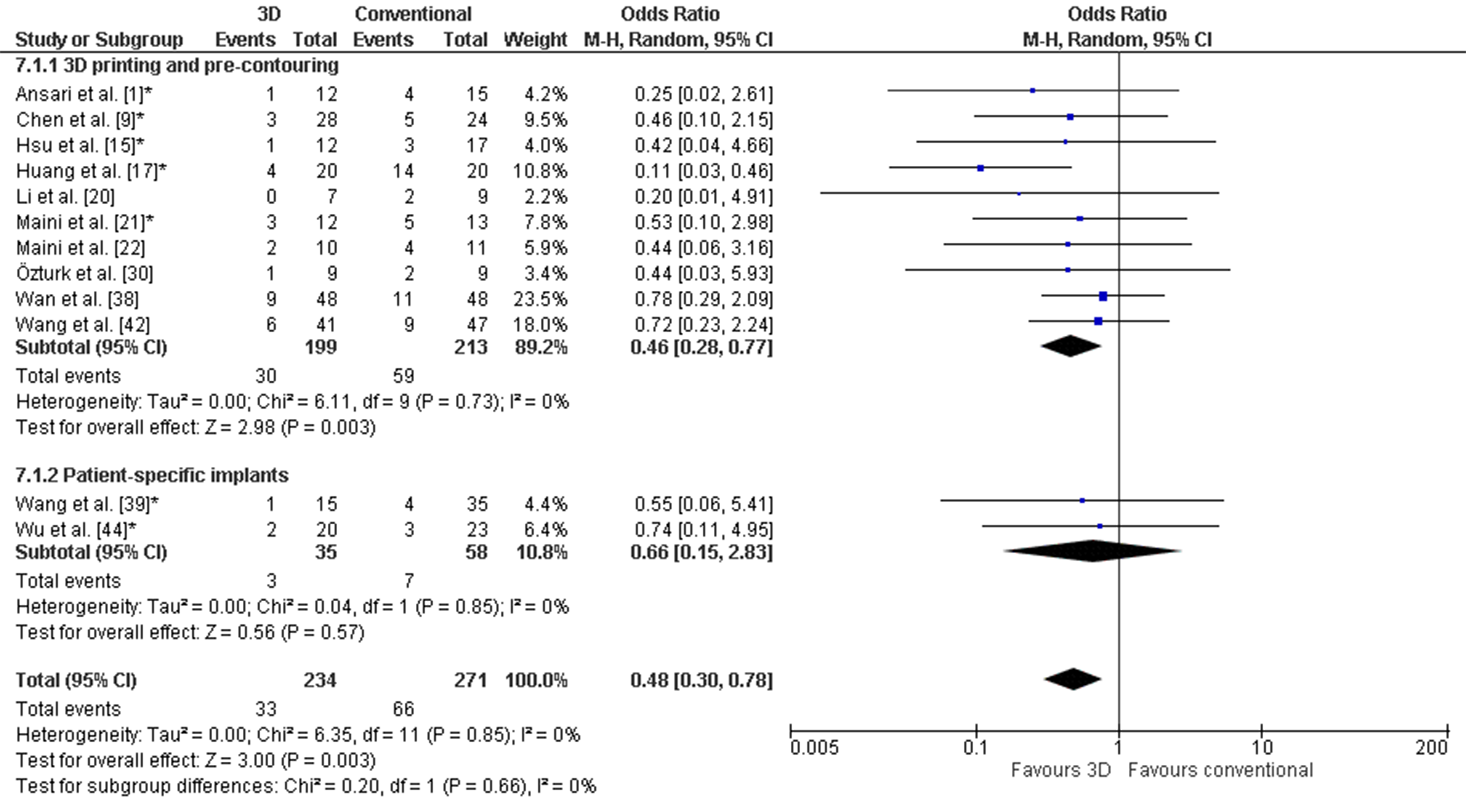 Figure 6. Forest plot of the postoperative reduction, where the events indicate a poor reduction. *: Good-quality study.
Figure 6. Forest plot of the postoperative reduction, where the events indicate a poor reduction. *: Good-quality study.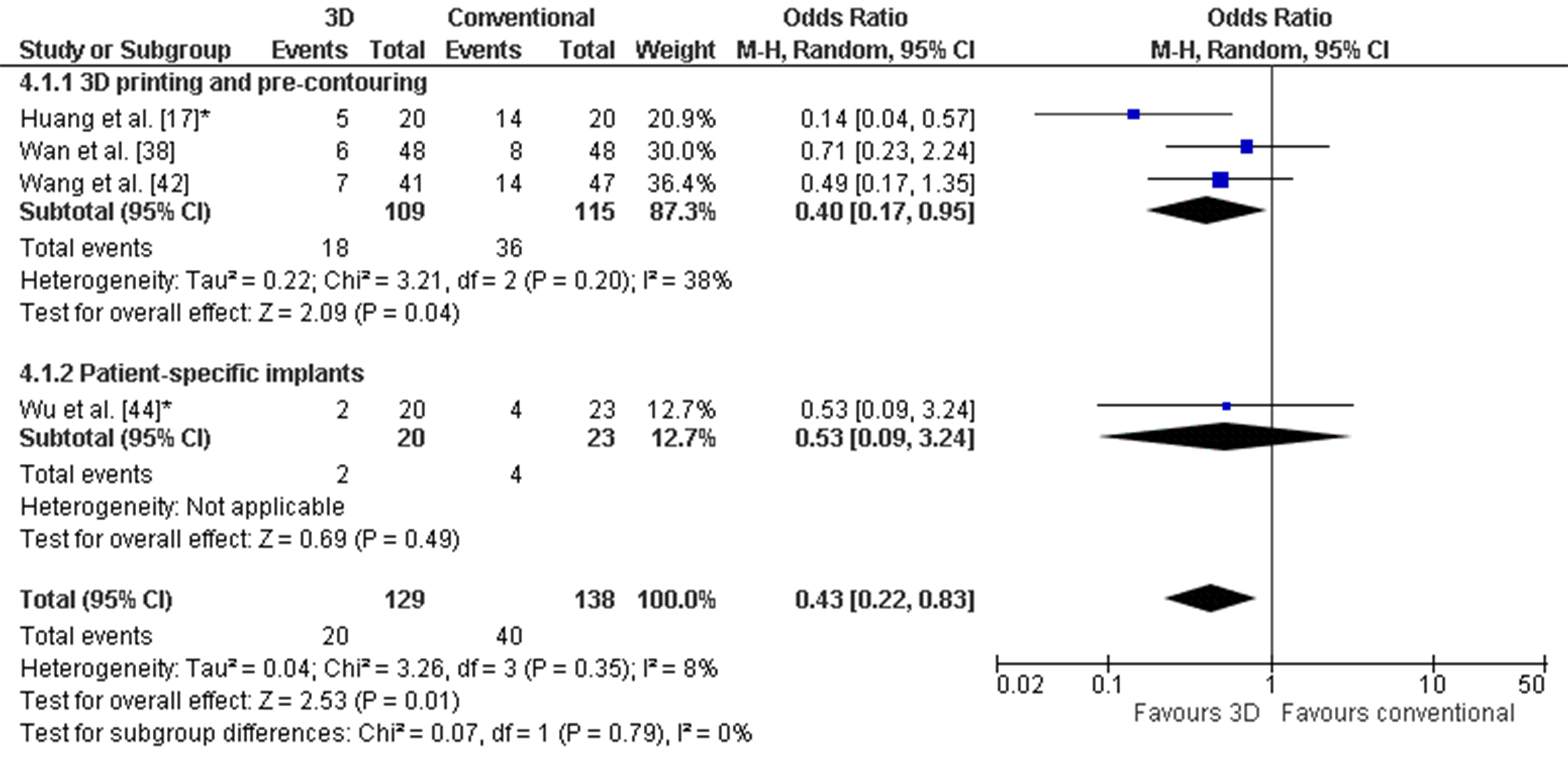 Figure 7. Forest plot of the functional outcome, where the events indicate a poor functional outcome. The Harris Hip score was used by Huang et al. [38] and Wan et al. [46]. The Merle d’Aubigné score was used by Wang et al. [32] and Wu et al. [34]. *: Good-quality study.
Figure 7. Forest plot of the functional outcome, where the events indicate a poor functional outcome. The Harris Hip score was used by Huang et al. [38] and Wan et al. [46]. The Merle d’Aubigné score was used by Wang et al. [32] and Wu et al. [34]. *: Good-quality study.4. Discussion
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jpm11100966
References
- Rinne, P.P.; Laitinen, M.K.; Huttunen, T.; Kannus, P.; Mattila, V.M. The incidence and trauma mechanisms of acetabular fractures: A nationwide study in Finland between 1997 and 2014. Injury 2017, 48, 2157–2161.
- Verbeek, D.O.; van der List, J.P.; Tissue, C.M.; Helfet, D.L. Predictors for Long-Term Hip Survivorship Following Acetabular Fracture Surgery—Importance of Gap Compared with Step Displacement. J. Bone Jt. Surg. 2018, 100, 922–929.
- Scheinfeld, M.H.; Dym, A.A.; Spektor, M.; Avery, L.L.; Dym, R.J.; Amanatullah, D.F. Acetabular fractures: What radiologists should know and how 3D CT can aid classification. RadioGraphics 2015, 35, 555–577.
- Brouwers, L.; Pull ter Gunne, A.F.; de Jongh, M.A.C.; van der Heijden, F.H.W.M.; Leenen, L.P.H.; Spanjersberg, W.R.; van Helden, S.H.; Verbeek, D.O.; Bemelman, M.; Lansink, K.W.W. The value of 3D printed models in understanding acetabular fractures. 3D Print. Addit. Manuf. 2018, 5, 37–46.
- Awan, O.A.; Sheth, M.; Sullivan, I.; Hussain, J.; Jonnalagadda, P.; Ling, S.; Ali, S. Efficacy of 3D Printed Models on Resident Learning and Understanding of Common Acetabular Fracturers. Acad. Radiol. 2019, 26, 130–135.
- Manganaro, M.S.; Morag, Y.; Weadock, W.J.; Yablon, C.M.; Gaetke-Udager, K.; Stein, E.B. Creating Three-dimensional Printed Models of Acetabular Fractures for Use as Educational Tools. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2017, 37, 871–880.
- Wang, H.; Lyu, F.; Sugand, K.; Wong, S.; Lin, Y.; Wang, Q. Learning Acetabular Fracture Classification using a Three-Dimensional Interactive Software: A Randomized Controlled Trial. Anat. Sci. Educ. 2018, 9, 1–9.
- Zheng, Y.X.; Yu, D.F.; Zhao, J.G.; Wu, Y.L.; Zheng, B. 3D Printout Models vs. 3D-Rendered Images: Which Is Better for Preoperative Planning? J. Surg. Educ. 2016, 73, 518–523.
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 1–22.
- Citak, M.; Gardner, M.J.; Kendoff, D.; Tarte, S.; Krettek, C.; Nolte, L.P.; Hüfner, T. Virtual 3D planning of acetabular fracture reduction. J. Orthop. Res. 2008, 26, 547–552.
- Hu, Y.; Li, H.; Qiao, G.; Liu, H.; Ji, A.; Ye, F. Computer-assisted virtual surgical procedure for acetabular fractures based on real CT data. Injury 2011, 42, 1121–1124.
- Fornaro, J.; Keel, M.; Harders, M.; Marincek, B.; Szekely, G.; Frauenfelder, T. An interactive surgical planning tool for acetabular fractures: Initial results. J. Orthop. Surg. Res. 2010, 5, 50.
- Upex, P.; Jouffroy, P.; Riouallon, G. Application of 3D printing for treating fractures of both columns of the acetabulum: Benefit of pre-contouring plates on the mirrored healthy pelvis. Orthop. Traumatol. Surg. Res. 2017, 103, 331–334.
- Yu, A.W.; Duncan, J.M.; Daurka, J.S.; Lewis, A.; Cobb, J. A feasibility study into the sse of three-dimensional printer modelling in acetabular fracture surgery. Adv. Orthop. 2015, 2015, 1–4.
- Chana-Rodríguez, F.; Mananes, R.P.; Rojo-Manaute, J.; Gil, P.; Martínez-Gómiz, J.M.; Vaquero-Martín, J. 3D surgical printing and pre contoured plates for acetabular fractures. Injury 2016, 47, 2507–2511.
- Merema, B.J.; Kraeima, J.; ten Duis, K.; Wendt, K.W.; Warta, R.; Vos, E.; Schepers, R.H.; Witjes, M.J.H.; IJpma, F.F.A. The design, production and clinical application of 3D patient-specific implants with drilling guides for acetabular surgery. Injury 2017, 48, 2540–2547.
- Wang, D.; Wang, Y.; Wu, S.; Lin, H.; Yang, Y.; Fan, S.; Gu, C.; Wang, J.; Song, C. Customized a Ti6Al4V bone plate for complex pelvic fracture by selective laser melting. Materials 2017, 10, 35.
- Brown, G.A.; Milner, B.; Firoozbakhsh, K. Application of computer-generated stereolithography and interpositioning template in acetabular fractures: A report of eight cases. J. Orthop. Trauma 2002, 16, 347–352.
- Meesters, A.M.L.; Assink, N.; Ten Duis, K.; Fennema, E.M.; Kraeima, J.; Witjes, M.J.H.; de Vries, J.P.P.M.; Stirler, V.M.A.; Ijpma, F.F.A. Accuracy of patient-specific drilling guides in acetabular fracture surgery: A human cadaver study. J. Pers. Med. 2021, 11, 763.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 8, 336–341.
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10.
- Law, M.; Stewart, D.; Pollock, N.; Letts, L.; Bosch, J.; Westmoreland, M. Guidelines for Critical Review form—Quantitative Studies. 1998. Available online: https://healthsci.mcmaster.ca/docs/librariesprovider130/default-document-library/guidelines-for-critical-review-form-quantiative-studies-english.pdf?sfvrsn=ee9f6c19_2 (accessed on 1 March 2021).
- Verbeek, D.O.; van der List, J.P.; Moloney, G.B.; Wellman, D.S.; Helfet, D.L. Assessing postoperative reduction following acetabular fracture surgery: A standardized digital CT-based method. J. Orthop. Trauma 2018, 32, e284–e288.
- Matta, J.M. Fractures of the acetabulum: Accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J. Bone Jt. Surg. 1996, 78, 1632–1645.
- Banaszkiewicz, P.A. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty: An End-Result Study Using a New Method of Result Evaluation. In Classic Papers in Orthopaedics, 1st ed.; Springer: London, UK, 2014; pp. 13–17. ISBN 9781447154518.
- Banaszkiewicz, P.A. Functional Results of Hip Arthroplasty with Acrylic Prosthesis. In Classic Papers in Orthopaedics, 1st ed.; Springer: London, UK, 2014; pp. 19–22. ISBN 9781447154518.
- Harris, W. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty: An End-Result Study Using a New Method of Result Evaluation. J. Bone Jt. Surg. 1969, 51, 737–755.
- D’Aubigne, R.; Postel, M. Functional Results of Hip Arthroplasty with Acrylic Prosthesis. J. Bone Jt. Surg. 1954, 36, 451–475.
- Xu, M.; Zhang, L.-H.; Zhang, Y.-Z.; Zhang, L.-C.; He, C.-Q.; Wang, Y.; Tang, P.-F. Custom-made locked plating for acetabular fracture: A pilot study in 24 consecutive cases. Orthopedics 2014, 37, e660–e670.
- Ansari, S.; Barik, S.; Singh, S.K.; Sarkar, B.; Goyal, T.; Kalia, R.B. Role of 3D printing in the management of complex acetabular fractures: A comparative study. Eur. J. Trauma Emerg. Surg. 2020.
- Chen, K.; Yang, F.; Yao, S.; Xiong, Z.; Sun, T.; Zhu, F.; Telemacque, D.; Drepaul, D.; Ren, Z.; Guo, X. Application of computer-assisted virtual surgical procedures and three-dimensional printing of patient-specific pre-contoured plates in bicolumnar acetabular fracture fixation. Orthop. Traumatol. Surg. Res. 2019, 105, 877–884.
- Wang, P.; Kandemir, U.; Zhang, B.; Fei, C.; Zhuang, Y.; Zhang, K. The effect of new preoperative preparation method compared to conventional method in complex acetabular fractures: Minimum 2-year follow-up. Arch. Orthop. Trauma Surg. 2020, 141, 215–222.
- Weidert, S.; Andress, S.; Linhart, C.; Suero, E.M.; Greiner, A.; Böcker, W.; Kammerlander, C.; Becker, C.A. 3D printing method for next-day acetabular fracture surgery using a surface filtering pipeline: Feasibility and 1-year clinical results. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 565–575.
- Wu, H.-Y.; Shao, Q.-P.; Song, C.-J.; Shang, R.-R.; Liu, X.-M.; Cai, X.-H. Personalized Three-Dimensional Printed Anterior Titanium Plate to Treat Double-Column Acetabular Fractures: A Retrospective Case-Control Study. Orthop. Surg. 2020, 12, 1212–1222.
- Yu, C.; Yu, W.; Mao, S.; Zhang, P.; Zhang, X.; Zeng, X.; Han, G. Traditional three-dimensional printing technology versus three-dimensional printing mirror model technology in the treatment of isolated acetabular fractures: A retrospective analysis. J. Int. Med. Res. 2020, 48, 0300060520924250.
- Zeng, C.; Xing, W.; Wu, Z.; Huang, H.; Huang, W. A combination of three-dimensional printing and computer-assisted virtual surgical procedure for preoperative planning of acetabular fracture reduction. Injury 2016, 47, 2223–2227.
- Zou, R.; Wu, M.; Guan, J.; Xiao, Y.; Chen, X. Therapeutic Effect of Acetabular Fractures Using the Pararectus Approach Combined with 3D Printing Technique. Orthop. Surg. 2020, 12, 1854–1858.
- Huang, J.-H.; Liao, H.; Tan, X.-Y.; Xing, W.-R.; Zhou, Q.; Zheng, Y.-S.; Cao, H.-Y.; Zeng, C.-J. Surgical treatment for both-column acetabular fractures using pre-operative virtual simulation and three-dimensional printing techniques. Chin. Med. J. 2020, 133, 395–401.
- IJpma, F.F.A.; Meesters, A.M.L.; Merema, B.B.J.; Duis, K.; De Vries, J.P.M.; Banierink, H.; Wendt, K.W.; Kraeima, J.; Witjes, M.J.H. Feasibility of Imaging-Based 3-Dimensional Models to Design Patient-Specific Osteosynthesis Plates and Drilling Guides. JAMA Netw. Open 2021, 4, 1–13.
- Downey, C.; McCarrick, C.; Fenelon, C.; Murphy, E.P.; O’Daly, B.J.; Leonard, M. A novel approach using 3-D printing in the Irish National Centre for pelvic and acetabular surgery. Ir. J. Med. Sci. 2020, 189, 219–228.
- Hsu, C.-L.; Chou, Y.-C.; Li, Y.-T.; Chen, J.-E.; Hung, C.-C.; Wu, C.-C.; Shen, H.-C.; Yeh, T.-T. Pre-operative virtual simulation and three-dimensional printing techniques for the surgical management of acetabular fractures. Int. Orthop. 2019, 43, 1969–1976.
- Li, Y.T.; Hung, C.C.; Chou, Y.C.; Chen, J.E.; Wu, C.C.; Shen, H.C.; Yeh, T. Te Surgical treatment for posterior dislocation of hip combined with acetabular fractures using preoperative virtual simulation and three-dimensional printing model-assisted precontoured plate fixation techniques. Biomed Res. Int. 2019, 2019, 3971571.
- Maini, L.; Sharma, A.; Jha, S.; Sharma, A.; Tiwari, A. Three-dimensional printing and patient-specific pre-contoured plate: Future of acetabulum fracture fixation? Eur. J. Trauma Emerg. Surg. 2018, 44, 215–224.
- Maini, L.; Verma, T.; Sharma, A.; Sharma, A.; Mishra, A.; Jha, S. Evaluation of accuracy of virtual surgical planning for patient-specific pre-contoured plate in acetabular fracture fixation. Arch. Orthop. Trauma Surg. 2018, 138, 495–504.
- Öztürk, A.M.; Süer, O.; Şirintürk, S.; Aktuğlu, K.; Govsa, F.; Özer, M.A. A retrospective comparison of the conventional versus three-dimensional printed model-assisted surgery in the treatment of acetabular fractures. Acta Orthop. Traumatol. Turc. 2020, 54, 385–393.
- Wan, L.; Zhang, X.; Zhang, S.; Li, K.; Cao, P.; Li, J.; Wu, G. Clinical feasibility and application value of computer virtual reduction combined with 3D printing technique in complex acetabular fractures. Exp. Ther. Med. 2019, 3630–3636.
- Wang, C.; Chen, Y.; Wang, L.; Wang, D.; Gu, C.; Lin, X.; Liu, H.; Chen, J.; Wen, X.; Liu, Y.; et al. Three-dimensional printing of patient-specific plates for the treatment of acetabular fractures involving quadrilateral plate disruption. BMC Musculoskelet. Disord. 2020, 21, 1–9.
- Meesters, A.M.L.; Kraeima, J.; Banierink, H.; Slump, C.H.; de Vries, J.P.P.M.; ten Duis, K.; Witjes, M.J.H.; IJpma, F.F.A. Introduction of a three-dimensional computed tomography measurement method for acetabular fractures. PLoS ONE 2019, 14, e0218612.
- Verbeek, D.O.; van der List, J.P.; Villa, J.C.; Wellman, D.S.; Helfet, D.L. Postoperative CT is superior for acetabular fracture reduction assessment and reliably predicts hip survivorship. J. Bone Jt. Surg. 2017, 99, 1745–1752.
- Tannast, M.; Najibi, S.; Matta, J.M. Two to twenty-year survivorship of the hip in 810 patients with operatively treated acetabular fractures. J. Bone Jt. Surg. 2012, 94-A, 1559–1567.
- Banierink, H.; ten Duis, K.; Wendt, K.; Heineman, E.; IJpma, F.; Reininga, I. Patient-reported physical functioning and quality of life after pelvic ring injury: A systematic review of the literature. PLoS ONE 2020, 15, e0233226.
- Meesters, A.M.L.; ten Duis, K.; Banierink, H.; Stirler, V.M.A.; Wouters, P.C.R.; Kraeima, J.; de Vries, J.P.P.M.; Witjes, M.J.H.; IJpma, F.F.A. What Are the Interobserver and Intraobserver Variability of Gap and Stepoff Measurements in Acetabular Fractures? Clin. Orthop. Relat. Res. 2020, 478, 2801–2808.
