Asexual Epichloë are obligate fungal mutualists that form symbiosis with many temperate grass species, providing several advantages to the host. These advantages include protection against vertebrate and invertebrate herbivores (i.e., grazing livestock and invertebrate pests, respectively), improved resistance to phytopathogens, increased adaptation to drought stress, nutrient deficiency, and heavy metal-containing soils. Selected Epichloë strains are utilised in agriculture mainly for their pest resistance traits, which are moderated via the production of Epichloë-derived secondary metabolites. For pastoral agriculture, the use of these endophyte infected grasses requires the balancing of protection against insect pests with reduced impacts on animal health and welfare.
- alkaloids
- animal toxicosis
- biocontrol
- endophyte
- fescue
- ryegrass
1. History
Microbial endophytes, primarily comprising archaea, bacteria, fungi, or viruses, are associated with most plant species [1][2]. The term ‘endophyte’ was derived from the Greek words ‘endon’ (within) and ‘phyton’ (plant) [3], and initially included both pathogenic and beneficial microorganisms [4]. However, the term endophyte has now become synonymous with mutualism in reference to microbes that spend all or part of their life cycle within the plant host while causing no apparent disease symptoms [5][6], and provides a net benefit outcome to both itself and the host plant [7].
Asexual Epichloë endophytes (previously belonging to the taxonomic genus Neotyphodium [8]) were identified in the 1980/90s as the cause of two economically important diseases that affected livestock that grazed fescue in the USA and perennial ryegrass in New Zealand, namely fescue toxicosis [9] and ryegrass staggers [10], respectively (Figure 1). These obligate symbionts are mutualistic, relying on the host plant for their growth, survival, and transmission through hyphal colonisation of the host’s seed [11]. These endophytes exhibit a degree of host-specificity within the cool-season grasses of the Pooideae, whereby Epichloë species are naturally restricted to a host grass genus or closely related genera within a grass tribe [12][13][14]. Asexual Epichloë spend their entire life cycle within the plant host growing systemically within shoot tissues between plant cells [15][16][17] (Figure 2). However, their bioactivity towards certain pests in the rhizosphere [18] can be attributed to the mobility of fungal secondary metabolites in the roots, produced during the symbiosis, within the plant vascular system [15][16].
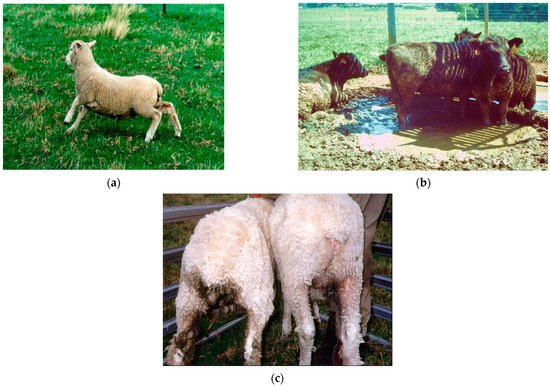

Epichloë-derived secondary metabolites protect the host plant from herbivores—both vertebrates and invertebrates. However, the effect on ruminants and several non-ruminants including horses, camels, white rhinoceros, and alpacas [19] can be detrimental and, when first discovered, removal of these endophytes from grasses was considered the best solution. However, in many temperate regions of the world, such as New Zealand, these Epichloë endophytes are essential for pasture persistence. Novel endophyte strains have now been identified and commercialised that provide the host grass with tolerance/resistance to pests, diseases, and some abiotic stresses while reducing or eliminating the debilitating animal health and welfare issues [20]. These endophytes can be transferred between plants through artificial infection [21].
2. Epichloë Endophytes—A Necessity for the Pastoral Industry?
2.1. Taxonomy and Distribution
Epichloë fungi are found in the Clavicipitaceae family [22]. As a result of taxonomists being required to have a single genus name for all stages of the development of a fungal species [23], the Epichloë genus contains both sexual (teleomorph) and asexual (anamorph) forms. The latter had previously been classified as Neotyphodium [8]. More than 100 cool-temperate grass species host Epichloë, with the majority originating from Europe and Asia, and with many fewer from Australasia, sub-Saharan Africa, and South America (Table 1).
Asexual Epichloë strains are either hybrid as a result of a cross between two or more species, or are non-hybrid [14][24]. While all hybrid types are asexual, those classified as non-hybrid types are also incapable of sexual reproduction [25][26]. Compared with other fungal genera, Epichloë has the greatest number of interspecific hybrids [27].
| Grass Genus (Common Names) | Epichloë Species | Region | Reference |
|---|---|---|---|
| Achnatherum | E. gansuensis, E. sibirica; E. chisosa; E. inebrians; E. funkii | Asia | [8][24][28][29] |
| Agropyron | E. bromicola | Europe/North Africa | [30] |
| Agrostis (browntop) | E. baconii, E. amarillans | Europe/North Africa | [31] |
| Ammophila | E. amarillans | North America | [32] |
| Anthoxanthum | E. typhina | Europe/North Africa | [31] |
| Brachyelytrum | E. brachyelytri | Europe/North Africa; North America | [31][33] |
| Brachypodium | E. sylvatica; E. typhina; E. bromicola | Europe/North Africa; Asia | [29][31] |
| Briza | E. tembladerae | South America | [34] |
| Bromus | E. bromicola; E. cabralii; E. typhina subsp. poae var.aonikenkana ; E. typhina; E. tembladerae; E. pampeana | Europe/North Africa; Asia; North America; South America | [8][29][31][34][35][36][37] |
| Calamagrostis | E. stromatolonga | Asia | [29] |
| Cinna | E. schardlii | North America | [38] |
| Dactylis (cocksfoot) | E. typhina | Europe/North Africa | [31] |
| Dichelachne | E. australiensis | New Zealand | [39] |
| Echinopogon | E. australiensis; E. aotearoae | Australia; New Zealand | [40][41] |
| Elymus | E. elymi; E. bromicola; E. canadensis | Europe/North Africa; Asia; North America | [8][24][29][31][33][42] |
| Elytrigia | E. spp. | Asia | [29] |
| Festuca (fescue) | E. coenophiala; E. festucae; E. uncinata; E. siegelii; E. sinofestucae; E. typhinum var. huerfana, E. tembladerae | Europe/North Africa; Asia; North America; South America | [29][31][43][44][45] |
| Glyceria | E. glyceriae | Europe/North Africa; North America | [31][33] |
| Holcus | E. typhina subsp. clarkii; E. mollis | Europe/North Africa | [8][31][46] |
| Hordelymus | E. disjuncta, E. danica, E. hordelymi, E. sylvatica subsp. pollinensis, | Europe/North Africa | [8][26] |
| Hordeum | E. tembladerae, E. amarillans, E. typhina hybrids | South America | [47] |
| Lolium (ryegrass) | E. occultans; E. typhina var. canariensis; E. hybrida; E. festucae var. lolii, E. typhina, | Europe/North Africa | [8][27][31][48] |
| Leymus | E. bromicola | Europe/North Africa; Asia | [29][31] |
| Melica | E. melicicola; E. guerinii; E. tembladerae | South America; Sub-Saharan Africa; South America | [8][24][34][41] |
| Phleum (timothy) | E. typhina; E. cabralii; E. tembladerae | Europe/North Africa; South America | [8][31][34][37] |
| Poa | E. typhina; E. liyangensis; E. alsodes; E. typhina subsp. poae; E. tembladerae; E. novae-zelandiae | Europe/North Africa; Asia; North America; South America; New Zealand | [31][34][39][43][49] |
| Roegneria | E. sinica; E. bromicola | Asia | [29][50] |
| Sphenopholis | E. amarillans | Europe/North Africa | [31] |
| Stipa | E. spp. | Asia | [29] |
2.2. Life Cycle
In planta, asexual Epichloë spp. complete their entire life cycle within the host plant’s tissues (Figure 3). Dissemination of Epichloë to the next plant generation occurs through vertical transmission of fungal hyphae via the seed of the host plant [51] and is moderated by the compatibility of the endophyte strain with the host’s genetics. Asexual Epichloë generally do not produce spores except when grown axenically within a laboratory environment [52].
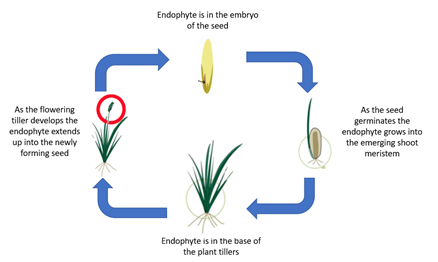
Figure 3. Schematic diagram of the asexual Epichloë endophytic life cycle in cool-season grasses of the Pooideae.
When used in managed pastoral ecosystems, viability of the endophyte in seed can be threatened if the seed is stored at ambient temperatures and humidity [53]. However, storage of seed with a moisture level below 8% at temperatures below 5 °C and relative humidity of less than 30% will ensure long term endophyte viability [54].
2.3. Secondary Metabolite Bioactivity and its Consequences
The impact of Epichloë in natural and agricultural ecosystems is largely driven by chemistry. The genome of the endophyte strain determines the types (quality) of secondary metabolites expressed, but the host plant genome largely regulates the amount (quantity) [55][56]. Considerable information is known about four types of these secondary metabolite compounds (Figure 4) [55][56][57]:
- Ergot alkaloids—can be divided into four groups based on their chemical structure: clavines (e.g., chanoclavine, agroclavine), lysergic acid, lysergic acid amides (e.g., ergonovine, ergine), and ergopeptines (e.g., ergovaline, ergotamine, ergocornine, ergocristine, ergosine, ergocryptine).
- Indole diterpenoids—lolitrems, epoxyjanthitrems, terpendoles, paxilline.
- Lolines—N-formyl loline (NFL), N-acetyl loline (NAL), N-acetylnorloline (NANL).
- Pyrrolopyrazine—peramine.
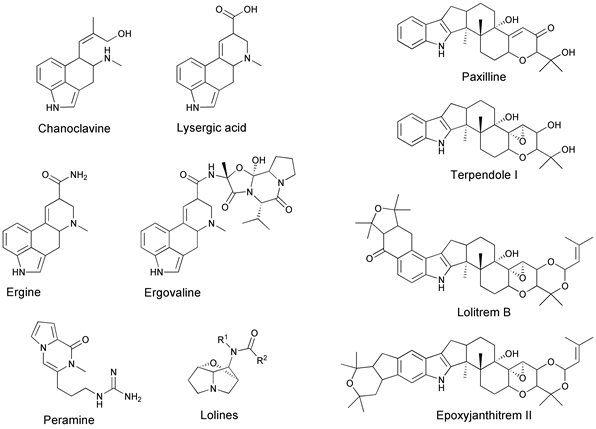
Figure 4. Molecular structures of the known secondary metabolites produced by Epichloë, in planta. Ergot alkaloids include chanoclavine, lysergic acid, ergine, and ergovaline; indole diterpenes include paxilline, terpendole I, lolitrem B, and epoxyjanthitrem II; peramine; and the lolines. (Figure provided courtesy of W. Mace).
Seasonal climatic effects can also have an overriding effect on the concentrations of secondary metabolites expressed [58][59][60]. These tend to be higher in the warmer drier periods of summer and autumn and lower through the cooler winter period (Figure 5).
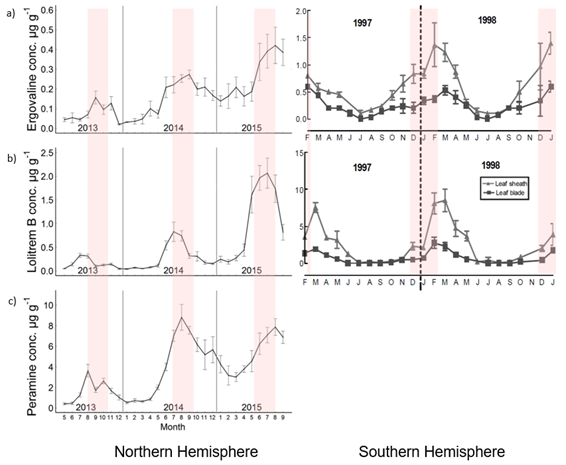
Figure 5. Seasonal trends in expression of ergovaline (a), lolitrem B (b), and peramine (c). Red shaded area represents summer months for the Northern and Southern Hemisphere. Figures modified from Fuchs et al [61] and Watson et al [62].
Secondary metabolite distribution within the plant can vary with compounds and among host species. In ryegrass, ergovaline is concentrated in the stem and basal leaf sheath of intermediate aged tillers, lolitrem B accumulates in older tissues, and peramine is distributed evenly across all leaf tissues [63]. In meadow fescue, lolines are found in both shoot and root tissues [64].
The bioactive impacts of Epichloë strains can vary depending on the quality and quantity of secondary metabolites expressed. Secondary metabolites causing most of the negative effects on mammals have been elucidated, but research continues to understand the role of beneficial secondary metabolites that provide advantages to host plant persistence (Table 2).
| Bioactivity Trait | Consequence | Causation | References |
|---|---|---|---|
| Disadvantageous bioactivity | |||
| Fescue toxicosis | Fescue foot, high core body temperature, increased respiration, low heart rate, altered fat metabolism, low serum prolactin, failure or reduced milk production produce milk, suppression of the immune system, reduced forage intake, low rate of weight gain, and reproductive problems. | Ergot alkaloids | [65][66][67] |
| Ryegrass staggers | Neurotoxic disease with symptoms ranging from slight muscular tremors through to staggering, and collapse. Can be associated with animal deaths. | Lolitrem B, other indole diterpenoids; sub-chronic threshold of 1.55 ppm for cattle and 2 to 2.5 ppm for sheep | [68][69][70] |
| Heat stress | Animals that are exposed to high concentrations of ergot alkaloids lose their ability to dissipate heat through restricted blood flow. | Ergovaline and other ergot alkaloids | [67][71][72] |
| Fecal soiling of the breech (dags) | Leads to higher incidence of myiasis (flystrike). | Ergovaline and lolitrem B | [73] |
| Reduced animal performance | Reduced liveweight gains and milk yields. | Ergovaline and lolitrem B | [73][74] |
| Ryegrass toxicosis | Can result in death through misadventure. | Documented only in Australia and likely to be a combination of ergot alkaloids and lolitrem B | [75][76][77] |
| Equine fescue oedema | Inappetence, depression, and subcutaneous oedema of the head, neck, chest, and abdomen in horses. | Only noted with endophytes in Mediterranean-type tall fescues | [78][79] |
| Reduced herbivore feeding | Deterrence to feeding. | Ergovaline and other ergot alkaloids | [80][81][82] |
| Livestock toxicosis (Australia, New Zealand) | Rare toxicosis in grazing livestock after Poa matthewsii and Echinopogon consumption. | Possible paxilline | [41][83] |
| Huecu toxicosis (Argentina) | Intoxication in grazing animals after Poa and Festuca grazing infected with E. tembladerae. | Indole-diterpenoid and ergot alkaloids | [84][85] |
| Sleepy grass toxicosis (USA) | Intoxication and narcosis in grazing livestock after Stipa robusta consumption. | Lysergic acid amide | [86][87] |
| Drunken horse grass toxicosis (Mongolia, China) | Intoxication and narcosis in horses, donkeys, sheep, goats, and cattle after Achnatherum inebrians consumption. | Ergot, ergonovine, lysergic acid, stipatoxin | [88] |
| Dronkgras toxicosis (South Africa) | Drunk-like behaviour of cattle, horses, donkeys consuming Melica decumbens. | Indole-diterpenoids | [89][90] |
| Advantageous bioactivity | |||
| Insect pest resistance |
Improved plant persistence and yield in presence of some insect pests. | Various alkaloids depending on insect species (Table 3) | [72][91][92] |
| Plant pathogen resistances |
Reduced incidence to some fungal diseases. | Many known, but possible effects of peroxidase activity, phenolic compounds and antifungal proteins | [72][93][94][95] |
| Drought (low water supply) tolerance | Improved tolerance of drought through moderation of stomatal conductance, enhanced osmoregulation. | Largely unknown, but compounds implicated include polyols; increased levels of sugars and proline | [96][97][98][99][100][101][102][103] |
| Allelopathy | Endophytic fescue plants reduce radicle elongation and growth of competing seedling species; but can detrimental on companion Trifolium species. | Total phenolic compound concentration was greater in endophytic than non-endophytic plants | [104] |
| Heavy metal tolerance | Improved growth in presence of cadmium, nickel. | Unknown; in case of cadmium improved translocation to shoot, but for nickel reduced translocation to shoot | [105][106] |
| Aluminium tolerance | Aluminium sequestration was greater on root surfaces and in root tissues of endophytic plants. | Increased exudation of phenolic-like compounds from roots of endophytic plants | [107] |
| Salinity tolerance | Improved leaf survival; changes of anatomical structures reducing water loss; and allowing water, nutrients, photosynthates translocation. | Unknown, but decreased sodium potassium and chlorine uptake | [108][109] |
| Nutrient uptake | Increased uptake of N and P from low levels of supply. | Unknown | [96][98][110][111] |
The mode of action of secondary metabolites against insect pests continues to be understood as new endophyte strains are discovered and improved control of insect pests is achieved (Table 3).
| Secondary Metabolite | Mode of Action | Invertebrates Affected | Reference |
|---|---|---|---|
| Ergot alkaloids | |||
| Ergovaline | Deterrent; anti-feeding; toxic | Argentine stem weevil (Listronotus bonariensis) adults; African black beetle (Heteronychus arator) adults; root aphid (Aploneura lentisci); Japanese beetle (Popillia japonica) larvae; black cutworm (Agrostis ipsilon); nematode (Pratylenchus scribneri) | [112][113][114][115][116] |
| Ergocryptine | Deterrent; anti-feeding; toxic | Argentine stem weevil adults and larvae; fall armyworm (Spodoptera frugiperda) larvae; nematode (Pratylenchus scribneri) | [117][118] |
| Indole diterpenoids | |||
| Lolitrem B | Deterrent; anti-feeding | Argentine stem weevil larvae; circumstantial effect on Paratylenchus nematode | [119] |
| Paxilline | Deterrent; anti-feeding | Argentine stem weevil larvae | [120] |
| Epoxyjanthitrems | Deterrent and toxic | Porina | [121] |
| Lolines | |||
| N-formyl loline, N-acetyl loline, N-acetylnorloline | Deterrent; anti-feeding | Grass grub (Costelytra giveni); horn flies (Haematobia irritans); African black beetle; field cricket (Gryllidae spp.); Japanese beetle (Popillia japonica) larvae | [122][123][124][125] |
| Deterrent and toxic | Argentine stem weevil larvae and adults; milkweed bug (Oncopeltus fasciatus); corn borer (Ostrinia nubilalis) larvae; aphid (Rhopalosiphum padi and Schizaphis graminum); fall armyworm (Spodoptera frugiperda) larvae; porina larvae (Wiseana ssp.); nematode (Pratylenchus scribneri) | [126][127][128][129][130] | |
| Pyrrolopyrazine | |||
| Peramine | Deterrent; anti-feeding | Argentine stem weevil adults and larvae | [120][131] |
| Not a deterrent, but disrupted development | Cutworm (Graphania mutans) | [118] | |
However, there are also many unidentified secondary metabolites that are known to exist through bioactivity against invertebrates that cannot be attributed to known chemistry. In addition, Epichloë endophytes boost the jasmonic acid pathway, which in turn, enhances the host plant’s immunity to chewing insects [132]. Grass emitted volatile organic compounds triggered by an aphid infestation can also be enhanced through Epichloë endophyte infection [133]. These volatiles have been shown to attract syrphid flies, which are natural enemies of aphids [134].
2.4. Application and Value to the Pastoral Industry
In a pastoral agriculture context, Epichloë endophytes are essential for temperate grass persistence in New Zealand, Australia, the USA, South America, and, to a lesser extent, Europe [135]. The absence of an Epichloë endophyte or use of an inappropriate strain can result in complete pasture loss, as shown in a comparative trial in the Waikato region of New Zealand (Figure 6). However, to ensure the disadvantageous impacts of Epichloë on animal health and welfare are minimised, strains of endophyte that do not express those secondary metabolites at levels causing these issues have been identified, isolated, and then re-inoculated into high yielding plant germplasm [72][136]. This has resulted in several novel Epichloë strain-host associations being commercialised [137][138][139][140][141][142]. Table 4 summarises the performance of these commercialised strains and their known chemistry.

Figure 6. The loss of persistence due to insect pests and drought on ryegrass with either no Epichloë endophyte or an ineffective strain on ryegrass persistence in the Waikato region of New Zealand.
| Epichloë Brand (or Strain) | Known Chemistry | Insect Pest Affects Significantly Reduced | Animal Performance | References |
|---|---|---|---|---|
| Ryegrass; endophytes are E. festucae var lolii, or E. festucae | ||||
| Nil endophyte | No chemistry | No insect pest protection | Excellent | [74][81][143][144] |
| AR1 | Peramine | Argentine stem weevil (larva), pasture mealybug (Balanococcus poae) | Excellent | [81] |
| AR37 | Epoxyjanthitrems | Argentine stem weevil (adult), pasture mealybug, porina, African black beetle, root aphid | Excellent, but minor staggers can occur with sheep/lambs | [145] |
| NEA (NEA2) | Low ergovaline and peramine, very low lolitrem B | Pasture mealybug, African black beetle | Excellent | [146][147] |
| NEA2 (mix of NEA2 and NEA6) | Medium ergovaline, medium-low peramine, very low lolitrem B | Argentine stem weevil, pasture mealybug, African black beetle, root aphid | Excellent, but lamb live weight gain could be reduced in extreme circumstances | [147][148][149][150][151] |
| NEA4 (mix of NEA2 and NEA3) | Medium ergovaline, medium-low peramine, very low lolitrem B | Argentine stem weevil, pasture mealybug, African black beetle | Excellent, but lamb live weight gain could be reduced in extreme circumstances | [147] |
| Standard endophyte | High ergovaline, peramine and lolitrem B | Argentine stem weevil, pasture mealybug, African black beetle; root aphid | Can cause ryegrass staggers in sheep and lambs, and significantly decrease lamb growth rates in summer and autumn, and significantly increase dags. In dairy cows, it has been shown to depress milksolids production through summer and autumn. |
[74][81][143][144][149][151] |
| Festulolium; endophyte is E. uncinatum | ||||
| U2 | Loline compounds NFL, NAL, and NANL | African black beetle, Argentine stem weevil, pasture mealybug, root aphid, grass grub, field crickets | Excellent | [124][152][153] |
| Tall fescue; endophytes are E. coenophiala | ||||
| Nil endophyte | No chemistry | No insect protection | Excellent | [154] |
| MaxQ II (USA); MaxP (NZ, Australia) (AR584) | Peramine, loline compounds NFL, NAL, and NANL | African black beetle, Argentine stem weevil, pasture mealybug, grass grub, root aphid, fall armyworm, corn flea beetle (Chaetocnema pulicaria), bird cherry-oat aphid (Rhopalosiphum padi), field crickets | Excellent | [155][156] |
| E34 | Low ergovaline | Not tested | Excellent; lowered blood serum prolactin levels | [156] |
The economic benefit of some of the more widely commercialised strains has been estimated. For example, the value to the New Zealand economy from AR37 has been calculated to be NZ$3.6 billion over the 20-year life of its patent [157]. In the USA, the positive financial benefits of MaxQ tall fescue have been demonstrated for beef cows, calves, and feeder cattle farming systems [158][159] and sheep farming systems [160].
3. Epichloë Endophytes—Applications Outside the Pastoral Industry
3.1. Application to the Turf Industry
For grassed areas such as airports, children’s playgrounds, sports turf, and parks, which exclude domesticated grazing animals, Epichloë endophytes that produce deterrent secondary metabolites such as ergot alkaloids, lolitrem B, and lolines have been deliberately used to discourage herbivores such as rabbits, granivorous birds, and rodents [161][162][163][164][165].
3.2. Application to Cereals
While Epichloë endophytes are not naturally found in modern cereals such as wheat (Triticum aestivum), barley (Hordeum vulgare), oats (Avena sativa), or rye (Secale cereale), they are found in wild grasses related to these species, namely from the genera Elymus and Hordeum [166]. Research has set out to determine whether Epichloë can provide the same benefits to cereals as they do to temperate grasses. Artificial inoculation of Epichloë strains from wild grasses into wheat and rye can result in stunted or dwarf phenotypes due to compatibility issues [167]. However, in outbreeding rye, the range of phenotypes can result in normal phenotypes, which, in the field, provide the host plant with increased fungal pathogen resistance and improved yields under some managements [168], and reduce damage from a range of insect pests [169][170]. In wheat, inoculations have been possible through using Chinese spring wheat addition lines [171].
4. Conclusions and Prospects
The mutualistic relationship between asexual Epichloë spp. and cool-season grasses is critical for the maintenance of temperate grass based pastoral agriculture in several countries. While it remains more of a biological curiosity in Europe, where this mutualistic association evolved between Epichloë and the host species of Lolium and Festuca, it is essential in many countries where these grasses were introduced due to the prevalence of both introduced and endemic insect pests and pathogens, and the compounding effect of drought and heat [72][103]. Changes in climate, management, and further pest incursions will continue to challenge the persistence of cool-season grasses making the reliance on the mutualism with Epichloë even more important. The ongoing search for novel endophytes with beneficial chemistry continues, but can now be supplemented with the use of new techniques such as gene editing to design endophyte strains for specific biotic and abiotic challenges [172][173]. The ultimate outcome will be the delivery of natural biocontrol options to protect grasses from pest and disease challenges, without the use of synthetic chemistry, and improve yield in drought conditions.
References
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.; Kloepper, J. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914.
- Arnold, A.E.; Herre, E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 2003, 95, 388–398.
- De Bary, A. Die Erscheinung der Symbiose: Vortrag Gehalten auf der Versammlung Deutscher Naturforscher und Aerzte zu Cassel; Verlage von Karl J. Trübner: Stassburg, France, 1879.
- Schulz, B.; Guske, S.; Dammann, U. Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227.
- Sinclair, J.; Cerkauskas, R. Latent infection vs. endophytic colonization by fungi. In Endophytic Fungi in Grasses and Woody Plants; Redlin, S.C., Carris, L.M., Eds.; American Phytopathological Society Press: St Paul, MN, USA, 1996; pp. 3–29.
- Saikkonen, K.; Wali, P.; Helander, M.; Faeth, S.H. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004, 9, 275–280.
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016, 92, fiw114.
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.J.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215.
- Schmidt, S.P.; Hoveland, C.S.; Clark, E.M.; Davis, N.D.; Smith, L.A.; Grimes, H.W.; Holliman, J.L. Association of an endophytic fungus with fescue toxicity in steers fed Kentucky 31 tall fescue seed or hay. J. Anim. Sci. 1982, 55, 1259–1263.
- Fletcher, L.R.; Harvey, I.C. An association of a Lolium endophyte with ryegrass staggers. N. Z. Vet. J. 1981, 29, 185–186.
- Zhang, W.; Card, S.D.; Mace, W.J.; Christensen, M.J.; McGill, C.R.; Matthew, C. Defining the pathways of symbiotic Epichloë colonization in grass embryos with confocal microscopy. Mycologia 2017, 109, 153–161.
- Schardl, C.L.; Craven, K.D.; Speakman, S.; Stromberg, A.; Lindstrom, A.; Yoshida, R. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst. Biol. 2008, 57, 483–498.
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343.
- Schardl, C.L. The epichloae, symbionts of the grass subfamily Poideae. Ann. Mo. Bot. Gard. 2010, 97, 646–665.
- Card, S.D.; Rolston, M.P.; Park, Z.; Cox, N.; Hume, D.E. Fungal endophyte detection in pasture grass seed utilising the infection layer and comparison to other detection techniques. Seed Sci. Technol. 2011, 39, 581–592.
- Christensen, M.; Voisey, C. The biology of the endophyte/grass partnership. N. Z. Grassl. Assoc. Res. Pract. Ser. 2006, 13, 123–133.
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.P.; Nickless, E.M.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93.
- Popay, A.J.; Bonos, S.A. Biotic Responses in Endophytic Grasses. In Neotyphodium in Cool-Season Grasses; Roberts, C.A., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; pp. 163–185.
- Hume, D.E.; Ryan, G.D.; Gibert, A.; Helander, M.; Mirlohi, A.; Sabzalian, M.R. Epichloë fungal endophytes for grassland ecosystems. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–305.
- Johnson, L.J.; De Bonth, A.C.M.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188.
- Latch, G.C.M.; Christensen, M.J. Artificial infection of grasses with endophytes. Ann. Appl. Biol. 1985, 107, 17–24.
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1992, 1, 150–162.
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Koeltz Botanical Books. Available online: https://www.iapt-taxon.org/nomen/main.php (accessed on 24 August 2021).
- Moon, C.D.; Craven, K.D.; Leuchtmann, A.; Clement, S.L.; Schardl, C.L. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol. Ecol. 2004, 13, 1455–1467.
- Schardl, C.; Craven, K. Interspecific hybridization in plant-associated fungi and oomycetes: A review. Mol. Ecol. 2003, 12, 2861–2873.
- Oberhofer, M.; Leuchtmann, A. Genetic diversity in epichloid endophytes of Hordelymus europaeus suggests repeated host jumps and interspecific hybridizations. Mol. Ecol. 2012, 21, 2713–2726.
- Campbell, M.A.; Tapper, B.A.; Simpson, W.R.; Johnson, R.D.; Mace, W.; Ram, A.; Lukito, Y.; Dupont, P.-Y.; Johnson, L.J.; Scott, D.B. Epichloë hybrida, sp. nov., an emerging model system for investigating fungal allopolyploidy. Mycologia 2017, 109, 715–729.
- Chen, L.; Li, X.; Li, C.; Swoboda, G.A.; Young, C.A.; Sugawara, K.; Leuchtmann, A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873.
- Song, H.; Nan, Z.; Song, Q.; Xia, C.; Li, X.; Yao, X.; Xu, W.; Kuang, Y.; Tian, P.; Zhang, Q. Advances in research on Epichloë endophytes in Chinese native grasses. Front. Microbiol. 2016, 7, 1399.
- Lembicz, M.; Górzyńska, K.; Leuchtmann, A. Choke disease caused by Epichloë bromicola in the grass Agropyron repens in Poland. Plant Dis. 2010, 94, 1372.
- Cagnano, G.; Roulund, N.; Jensen, C.S.; Forte, F.P.; Asp, T.; Leuchtmann, A. Large scale screening of Epichloë endophytes infecting Schedonorus pratensis and other forage grasses reveals a relation between microsatellite-based haplotypes and loline alkaloid levels. Front. Plant Sci. 2019, 10, 765.
- Drake, I.; White, J.F., Jr.; Belanger, F.C. Identification of the fungal endophyte of Ammophila breviligulata (American beachgrass) as Epichloë amarillans. PeerJ 2018, 6, e4300.
- Schardl, C.L.; Leuchtmann, A. Three new species of Epichloë symbiotic with North American grasses. Mycologia 1999, 91, 95–107.
- Iannone, L.J.; Novas, M.V.; Young, C.A.; De Battista, J.P.; Schardl, C.L. Endophytes of native grasses from South America: Biodiversity and ecology. Fungal Ecol. 2012, 5, 357–363.
- Gentile, A.; Rossi, M.S.; Cabral, D.; Craven, K.D.; Schardl, C.L. Origin, divergence, and phylogeny of Epichloë endophytes of native Argentine grasses. Mol. Phylogenet. Evol. 2005, 35, 196–208.
- Charlton, N.D.; Craven, K.D.; Afkhami, M.E.; Hall, B.A.; Ghimire, S.R.; Young, C.A. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes. FEMS Microbiol. Ecol. 2014, 90, 276–289.
- Mc Cargo, P.D.; Iannone, L.J.; Vignale, M.V.; Schardl, C.L.; Rossi, M.S. Species diversity of Epichloë symbiotic with two grasses from southern Argentinean Patagonia. Mycologia 2014, 106, 339–352.
- Ghimire, S.R.; Rudgers, J.A.; Charlton, N.D.; Young, C.; Craven, K.D. Prevalence of an intraspecific Neotyphodium hybrid in natural populations of stout wood reed (Cinna arundinacea L.) from eastern North America. Mycologia 2011, 103, 75–84.
- Leuchtmann, A.; Young, C.A.; Stewart, A.V.; Simpson, W.R.; Hume, D.E.; Scott, B. Epichloe novae-zelandiae, a new endophyte from the endemic New Zealand grass Poa matthewsii. N. Z. J. Bot. 2019, 57, 271–288.
- Miles, C.O.; Menna, M.E.d.; Jacobs, S.W.L.; Garthwaite, I.; Lane, G.A.; Prestidge, R.A.; Marshall, S.L.; Wilkinson, H.H.; Schardl, C.L.; Ball, O.J.P.; et al. Endophytic fungi in indigenous Australasian grasses associated with toxicity to livestock. Appl. Environ. Microbiol. 1998, 64, 601–606.
- Moon, C.D.; Miles, C.O.; Järlfors, U.; Schardl, C.L. The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the Southern Hemisphere. Mycologia 2002, 94, 694–711.
- Burr, K.; Mittal, S.; Hopkins, A.; Young, C. Characterisation of fungal endophytes present in Elymus canadensis (Canada wildrye). N. Z. Grassl. Assoc. Res. Pract. Ser. 2006, 13, 473–476.
- Cabral, D.; Iannone, L.J.; Stewart, A.V.; Novas, M.V. The distribution and incidence of Neotyphodium endophytes in native grasses from Argentina and its association with environmental factors. N. Z. Grassl. Assoc. Res. Pract. Ser. 2006, 13, 79–82.
- Craven, K.D.; Blankenship, J.D.; Leuchtmann, A.; Hignight, K.; Schardl, C.L. Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia 2001, 53, 44–73.
- Iannone, L.J.; Pinget, A.D.; Nagabhyru, P.; Schardl, C.L.; De Battista, J.P. Beneficial effects of Neotyphodium tembladerae and Neotyphodium pampeanum on a wild forage grass. Grass Forage Sci. 2012, 67, 382–390.
- Clay, K.; Brown, V.K. Infection of Holcus lanatus and H. mollis by Epichloë in experimental grasslands. Oikos 1997, 79, 363–370.
- Iannone, L.J.; Irisarri, J.G.N.; Mc Cargo, P.D.; Pérez, L.I.; Gundel, P.E. Occurrence of Epichloë fungal endophytes in the sheep-preferred grass Hordeum comosum from Patagonia. J. Arid Environ. 2015, 115, 19–26.
- Moon, C.D.; Scott, D.B.; Schardl, C.L.; Christensen, M.J. The evolutionary origins of Epichloe endophytes from annual ryegrasses. Mycologia 2000, 92, 1103–1118.
- Kang, Y.; Ji, Y.L.; Zhang, C.W.; Wang, Z.W. Neotyphodium sinicum, from several Roegneria species throughout China, provides insights into the evolution of asexual endophytes. Symbiosis 2011, 54, 37–45.
- Li, W.; Ji, Y.; Yu, H.; Wang, Z. A new species of Epichloë symbiotic with Chinese grasses. Mycologia 2006, 98, 560–570.
- Philipson, M.N. A symptomless endophyte of ryegrass (Lolium perenne) that spores on its host—A light microscope study. N. Z. J. Bot. 1989, 27, 513–519.
- Tadych, M.; Ambrose, K.V.; Bergen, M.S.; Belanger, F.C.; White Jr, J.F. Taxonomic placement of Epichloë poae sp. nov. and horizontal dissemination to seedlings via conidia. Fungal Divers. 2012, 54, 117–131.
- Hume, D.E.; Schmid, J.; Rolston, M.P.; Vijayan, P.; Hickey, M.J. Effect of climatic conditions on endophyte and seed viability in stored ryegrass seed. Seed Sci. Technol. 2011, 39, 481–489.
- Hume, D.E.; Card, S.D.; Rolston, M.P. Effects of storage conditions on endophyte and seed viability in pasture grasses. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013; pp. 405–408.
- Easton, H.S.; Latch, G.C.M.; Tapper, B.A.; Ball, O.J.P. Ryegrass host genetic control of concentrations of endophyte-derived alkaloids. Crop Sci. 2002, 42, 51–57.
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368.
- Schardl, C.L.; Young, C.A.; Faulkner, J.R.; Florea, S.; Pan, J. Chemotypic diversity of epichloae, fungal symbionts of grasses. Fungal Ecol. 2012, 5, 331–344.
- Agee, C.S.; Hill, N.S. Ergovaline variability in Acremonium-infected tall fescue due to environment and plant genotype. Crop Sci. 1994, 34, 221–226.
- Brosi, G.B.; McCulley, R.L.; Bush, L.P.; Nelson, J.A.; Classen, A.T.; Norby, R.J. Effects of multiple climate change factors on the tall fescue-fungal endophyte symbiosis: Infection frequency and tissue chemistry. New Phytol. 2011, 189, 797–805.
- Hennessy, L.M.; Popay, A.J.; Finch, S.C.; Clearwater, M.J.; Cave, V.M. Temperature and plant genotype alter alkaloid concentrations in ryegrass infected with an Epichloë endophyte and this affects an insect herbivore. Front. Plant Sci. 2016, 7, 1097.
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J. Plant age and seasonal timing determine endophyte growth and alkaloid biosynthesis. Fungal Ecol. 2017, 29, 52–58.
- Watson, R.H.; Keogh, R.G.; McDonald, M.F. Ewe reproductive performance and growth rate of suckling-lambs on endophyte-infected perennial ryegrass pasture. N. Z. Grassl. Assoc. Res. Pract. Ser. 1999, 7, 19–26.
- Ball, O.J.-P.; Barker, G.M.; Prestidge, R.A.; Lauren, D.R. Distribution and accumulation of the alkaloid peramine in Neotyphodium lolii-infected perennial ryegrass. J. Chem. Ecol. 1997, 23, 1419–1434.
- Patchett, B.J.; Chapman, R.B.; Fletcher, L.R.; Gooneratne, S.R. Root loline concentration in endophyte-infected meadow fescue (Festuca pratensis) is increased by grass grub (Costelytra zealandica) attack. N. Z. Plant Prot. 2008, 61, 210–214.
- Thompson, F.N.; Stuedemann, J.A. Pathophysiology of fescue toxicosis. Agric. Ecosyst. Environ. 1993, 44, 263–281.
- Roberts, C.; Andrae, J. Tall fescue toxicosis and management. Crop Manag. 2004, 3, 1–18.
- Strickland, J.R.; Aiken, G.E.; Klotz, J.L. Ergot alkaloid induced blood vessel dysfunction contributes to fescue toxicosis. Forage Grazinglands 2009, 7, 1–7.
- Di Menna, M.E.; Mortimer, P.H.; Prestidge, R.A.; Hawkes, A.D.; Sprosen, J.M. Lolitrem B concentrations, counts of Acremonium lolii hyphae, and the incidence of ryegrass staggers in lambs on plots of A. lolii-infected perennial ryegrass. N. Z. J. Agric. Res. 1992, 35, 211–217.
- Prestidge, R.A. Causes and control of perennial ryegrass staggers in New Zealand. Agric. Ecosyst. Environ. 1993, 44, 283–300.
- Duringer, J.M.; Blythe, L.L.; Estill, C.T.; Moon, A.; Murty, L.; Livesay, S.; Galen, A.; Craig, A.M. Determination of a sub-chronic threshold for lolitrem B and perennial ryegrass toxicosis in Angus cattle consuming endophyte-infected perennial ryegrass (Lolium perenne) straw over 64 days. Livest. Sci. 2021, 250, 104570.
- Klotz, J.; Nicol, A. Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism. Anim. Prod. Sci. 2016, 56, 1761–1774.
- Caradus, J.R.; Johnson, L.J. Epichloë fungal endophytes—From a biological curiosity in wild grasses to an essential component of resilient high performing ryegrass and fescue pastures. J. Fungi 2020, 6, 322.
- Fletcher, L.R.; Sutherland, B.L.; Fletcher, C.G. The impact of endophyte on the health and productivity of sheep grazing ryegrass based pastures. In Proceedings of the Ryegrass Endophyte—An Essential New Zealand Symbiosis; Grassland Research and Practice Series No. 7; New Zealand Grassland Association: Napier, New Zealand; pp. 11–17.
- Bluett, S.J.; Thom, E.R.; Clark, D.A.; Waugh, C.D. Effects of a novel ryegrass endophyte on pasture production, dairy cow milk production and calf liveweight gain. Aust. J. Exp. Agric. 2005, 45, 11–19.
- Van Heeswijck, R.; McDonald, G. Acremonium endophytes in perennial ryegrass and other pasture grasses in Australia and New Zealand. Aust. J. Agric. Res. 1992, 43, 1683–1709.
- Reed, K.F.M.; Page, S.W.; Lean, I.J. Perennial Ryegrass Toxicosis in Australia; Meat & Livestock Australia: North Sydney, Australia, 2005; p. 100.
- Reed, K.F.M.; Nie, Z.N.; Walker, L.V.; Mace, W.J.; Clark, S.G. Weather and pasture characteristics associated with outbreaks of perennial ryegrass toxicosis in southern Australia. Anim. Prod. Sci. 2011, 51, 738–752.
- Bourke, C.A.; Hunt, E.; Watson, R. Fescue-associated oedema of horses grazing on endophyte-inoculated tall fescue grass (Festuca arundinacea) pastures. Aust. Vet. J. 2009, 87, 492–498.
- Finch, S.; Munday, J.; Sutherland, B.; Vlaming, J.; Fletcher, L. Further investigation of equine fescue oedema induced by Mediterranean tall fescue (Lolium arundinaceum) infected with selected fungal endophytes (Epichloë coenophiala). N. Z. Vet. J. 2017, 65, 322–326.
- Cosgrove, G.P.; Anderson, C.B.; Berquist, T.R.N. Fungal endophyte effects on intake, health and liveweight gain of grazing cattle. N. Z. Grassl. Assoc. 1996, 57, 43–48.
- Fletcher, L.R. “Non-toxic” endophytes in ryegrass and their effect on livestock health and production. NZGA Res. Pract. Ser. 1999, 7, 133–139.
- Thom, E.R.; Waugh, C.D.; Minnee, E.M.K.; Waghorn, G.C. Effects of novel and wild-type endophytes in perennial ryegrass on cow health and production. N. Z. Vet. J. 2013, 61, 87–97.
- Seddon, H.; Carne, H. Staggers in Stock due to Rough-Bearded Grass (Echinopogon Ovatus); New South Wales Department of Agriculture Bulletin: Sydney, Australia, 1926; pp. 34–40.
- Pomilio, A.; Rofi, R.; Gambino, M.; Mazzini, C.; de Langenheim, R.D. The lethal principle of Poa huecu (coirón blanco): A plant indigenous to Argentina. Toxicon 1989, 27, 1251–1262.
- Miles, C.; Uzal, F.; Garthwaite, I.; Munday-Finch, S.; di Menna, M. Poa huecu and Festuca argentina; AgResearch: Hamilton, New Zealand, 1995; p. 20.
- Petroski, R.J.; Powell, R.G.; Clay, K. Alkaloids of Stipa robusta (sleepygrass) infected with an Acremonium endophyte. Nat. Toxins 1992, 1, 84–88.
- Shymanovich, T.; Saari, S.; Lovin, M.E.; Jarmusch, A.K.; Jarmusch, S.A.; Musso, A.M.; Charlton, N.D.; Young, C.A.; Cech, N.B.; Faeth, S.H. Alkaloid variation among epichloid endophytes of sleepygrass (Achnatherum robustum) and consequences for resistance to insect herbivores. J. Chem. Ecol. 2015, 41, 93–104.
- Miles, C.O.; Lane, G.A.; Menna, M.E.d.; Garthwaite, I.; Piper, E.L.; Ball, O.J.P.; Latch, G.C.M.; Allen, J.M.; Hunt, M.B.; Bush, L.P.; et al. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J. Agric. Food Chem. 1996, 44, 1285–1290.
- Russell, G.G.; Ellis, R. The genus Melica L. (Poaceae) in southern Africa. Bothalia 1982, 14, 37–44.
- Miles, C.; di Menna, M.; Kellerman, T.; Garthwaite, I.; Ball, O. Melica Decumbens; AgResearch: Hamilton, New Zealand, 1995; p. 20.
- Popay, A.J.; Rowan, D.D. Endophytic fungi as mediators of plant-insect interactions. In Insect-Plant Interactions 5; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 83–103.
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7.
- Wiewióra, B.; Żurek, G.; Żurek, M. Endophyte-mediated disease resistance in wild populations of perennial ryegrass (Lolium perenne). Fungal Ecol. 2015, 15, 1–8.
- Xia, C.; Li, N.; Zhang, Y.; Li, C.; Zhang, X.; Nan, Z. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073.
- Card, S.D.; Bastías, D.A.; Caradus, J.R. Antagonism to Plant Pathogens by Epichloë Fungal Endophytes—A Review. Plants 2021, 10, 1997.
- Bacon, C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993, 44, 123–141.
- Elmi, A.; West, C. Endophyte effects on tall fescue stomatal response, osmotic adjustment, and tiller survival. New Phytol. 1995, 131, 61–67.
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000, 40, 923–940.
- Hahn, H.; McManus, M.T.; Warnstorff, K.; Monahan, B.J.; Young, C.A.; Davies, E.; Tapper, B.A.; Scott, B. Neotyphodium fungal endophytes confer physiological protection to perennial ryegrass (Lolium perenne L.) subjected to a water deficit. Environ. Exp. Bot. 2008, 63, 183–199.
- He, L.; Hatier, J.; Card, S.; Matthew, C. Endophyte-infection reduces leaf dehydration of ryegrass and tall fescue plants under moderate water deficit. N. Z. Grassl. Assoc. 2013, 5–7.
- Nagabhyru, P.; Dinkins, R.D.; Wood, C.L.; Bacon, C.W.; Schardl, C.L. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol. 2013, 13, 127.
- Decunta, F.A.; Pérez, L.I.; Malinowski, D.P.; Molina-Montenegro, M.A.; Gundel, P.E. A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool-season grasses. Front. Plant Sci. 2021, 12, 380.
- Hewitt, K.G.; Popay, A.J.; Hofmann, R.W.; Caradus, J.R. Epichloë—A lifeline for temperate grasses under combined drought and insect pressure. Grass Res. 2021, 1, 7.
- Vázquez-de-Aldana, B.R.; Romo, M.; García-Ciudad, A.; Petisco, C.; García-Criado, B. Infection with the fungal endophyte Epichloë festucae may alter the allelopathic potential of red fescue. Ann. Appl. Biol. 2011, 159, 281–290.
- Ren, A.; Li, C.; Gao, Y. Endophytic fungus improves growth and metal uptake of Lolium arundinaceum Darbyshire ex. Schreb. Int. J. Phytoremediat. 2011, 13, 233–243.
- Mirzahossini, Z.; Shabani, L.; Sabzalian, M.R.; Sharifi-Tehrani, M. ABC transporter and metallothionein expression affected by NI and Epichloe endophyte infection in tall fescue. Ecotoxicol. Environ. Saf. 2015, 120, 13–19.
- Malinowski, D.P.; Belesky, D.P. Tall fescue aluminum tolerance is affected by Neotyphodium coenophialum endophyte. J. Plant Nutr. 1999, 22, 1335–1349.
- Reza Sabzalian, M.; Mirlohi, A. Neotyphodium endophytes trigger salt resistance in tall and meadow fescues. J. Plant Nutr. Soil Sci. 2010, 173, 952–957.
- Ren, A.; Wei, M.; Yin, L.; Wu, L.; Zhou, Y.; Li, X.; Gao, Y. Benefits of a fungal endophyte in Leymus chinensis depend more on water than on nutrient availability. Environ. Exp. Bot. 2014, 108, 71–78.
- Malinowski, D.P.; Belesky, D.P. Neotyphodium coenophialum-endophyte infection affects the ability of tall fescue to use sparingly available phosphorus. J. Plant Nutr. 1999, 22, 835–853.
- Wang, J.; Nan, Z.; Christensen, M.J.; Li, C. Glucose-6-phosphate dehydrogenase plays a vital role in Achnatherum inebrians plants host to Epichloë gansuensis by improving growth under nitrogen deficiency. Plant Soil 2018, 430, 37–48.
- Patterson, C.G.; Potter, D.A.; Fannin, F.F. Feeding deterrency of alkaloids from endophyte-infected grasses to Japanese beetle grubs. Entomol. Exp. Appl. 1991, 61, 285–289.
- Popay, A.J.; Wyatt, R.T. Resistance to Argentine stem weevil in perennial ryegrass infected with endophytes producing different alkaloids. N. Z. Plant Prot. 1995, 48, 229–236.
- Ball, O.J.-P.; Miles, C.O.; Prestidge, R.A. Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1997, 90, 1382–1391.
- Popay, A.J.; Gerard, P.J. Cultivar and endophyte effects on a root aphid, Aploneura lentisci, in perennial ryegrass. N. Z. Plant Prot. 2007, 60, 223–227.
- Potter, D.A.; Stokes, J.T.; Redmond, C.T.; Schardl, C.L.; Panaccione, D.G. Contribution of ergot alkaloids to suppression of a grass-feeding caterpillar assessed with gene-knockout endophytes in perennial ryegrass. Entomol. Exp. Appl. 2007, 126, 138–147.
- Clay, K.; Cheplick, G.P. Effect of ergot alkaloids from fungal endophyte-infected grasses on fall armyworm (Spodoptera frugiperda). J. Chem. Ecol. 1989, 15, 169–182.
- Dymock, J.; Rowan, D.; McGee, I. Effects of endophyte-produced mycotoxins on Argentine stem weevil and the cutworm Graphania mutans. In Proceedings of the 5th Australasian Grassland Invertebrate Ecology Conference, Victoria, Australia, 5–19 August 1988; pp. 35–43.
- Rowan, D.D.; Gaynor, D.L. Isolation of feeding deterrents against Argentine stem weevil from ryegrass infected with the endophyte Acremonium loliae. J. Chem. Ecol. 1986, 12, 647–658.
- Rowan, D.D. Lolitrems, peramine and paxilline: Mycotoxins of the ryegrass/endophyte interaction. Agric. Ecosyst. Environ. 1993, 44, 103–122.
- Popay, A.J.; Cotching, B.; Moorhead, A.; Ferguson, C.M. AR37 endophyte effects on porina and root aphid populations and ryegrass damage in the field. N. Z. Grassl. Assoc. 2012, 74, 165–170.
- Bryant, J.R.; Lambert, M.G.; Brazendale, R.; Holmes, C.W.; Fraser, T.J. Effects of integrated cropping and pasture renewal on the performance and profit of dairy farms. N. Z. Grassl. Assoc. 2010, 72, 29–34.
- Patchett, B.J.; Gooneratne, R.B.; Chapman, B.; Fletcher, L.R. Effects of loline-producing endophyte-infected meadow fescue ecotypes on New Zealand grass grub (Costelytra zealandica). N. Z. J. Agric. Res. 2011, 54, 303–313.
- Barker, G.M.; Patchett, B.J.; Cameron, N.E. Epichloë uncinata infection and loline content protect Festulolium grasses from crickets (Orthoptera: Gryllidae). J. Econ. Entomol. 2015, 108, 789–797.
- Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Bardehle, L.; Escobar-Bahamondes, P.; Ungerfeld, E. Antifeedant effects and repellent activity of loline alkaloids from endophyte-infected tall fescue against horn flies, Haematobia irritans (Diptera: Muscidae). Molecules 2021, 26, 817.
- Johnson, M.; Dahlman, D.; Siegel, M.; Bush, L.; Latch, G.; Potter, D.; Varney, D. Insect feeding deterrents in endophyte-infected tall fescue. Appl. Environ. Microbiol. 1985, 49, 568–571.
- Riedell, W.; Kieckhefer, R.; Petroski, R.; Powell, R. Naturally-occurring and synthetic loline alkaloid derivatives: Insect feeding behavior modification and toxicity. J. Entomol. Sci. 1991, 26, 122–129.
- Popay, A.J.; Lane, G.A. The effect of crude extracts containing loline alkaloids on two New Zealand insect pests. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000; pp. 471–475.
- Koppenhöfer, A.M.; Fuzy, E.M. Effects of turfgrass endophytes (Clavicipitaceae: Ascomycetes) on white grub (Coleoptera: Scarabaeidae) control by the entomopathogenic nematode Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae). Environ. Entomol. 2003, 32, 392–396.
- Jensen, J.G.; Popay, A.J.; Tapper, B.A. Argentine stem weevil adults are affected by meadow fescue endophyte and its loline alkaloids. N. Z. Plant Prot. 2009, 62, 12–18.
- Gaynor, D.; Rowan, D. Peramine—An Argentine stem weevil feeding deterrent from endophyte-infected ryegrass. In Proceedings of the 4th Australiasian Conference on Grassland Invertebrate Ecology Canterbury, Canterbury, New Zealand, 13–17 May 1985; pp. 338–343.
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë fungal endophytes and plant defenses: Not just alkaloids. Trends Plant Sci. 2017, 22, 939–948.
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol. 2019, 38, 98–103.
- Nelson, E.H.; Hogg, B.N.; Mills, N.J.; Daane, K.M. Syrphid flies suppress lettuce aphids. BioControl 2012, 57, 819–826.
- Easton, H.S.; Fletcher, L.R. The importance of endophyte in agricultural systems—Changing plant and animal productivity. In Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses, Christchurch, New Zealand, 25–28 March 2007; pp. 11–18.
- Caradus, J.R.; Johnson, L.J. Improved adaptation of temperate grasses through mutualism with fungal endophytes. In Endophyte biotechnology: Potential for Agriculture and Pharmacology; Schouten, A., Ed.; CABI: Wallingford, UK, 2019; pp. 85–108.
- Caradus, J. The commercial impact of Neotyphodium endophyte science and technology. In Proceedings of the 8th International Grass Endophyte Symposium, Lanzhou, China, 13–16 August 2012; pp. 13–16.
- Fletcher, L.R. Novel endophytes in New Zealand grazing systems: The perfect solution or a compromise? In Epichloae, Endophytes of Cool Season Grasses: Implications, Utilization and Biology; Young, C.A., Aiken, G.E., McCulley, R.L., Strickland, J.R., Schardl, C.L., Eds.; The Samuel Roberts Noble Foundation: Ardmore, OK, USA, 2012; pp. 5–13.
- Thom, E.R.; Popay, A.J.; Hume, D.E.; Fletcher, L.R. Evaluating the performance of endophytes in farm systems to improve farmer outcomes—A review. Crop Pasture Sci. 2012, 63, 927–943.
- Young, C.A.; Hume, D.E.; McCulley, R.L. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Sci. 2013, 91, 2379–2394.
- Hume, D.E.; Stewart, A.V.; Simpson, W.R.; Johnson, R.D. Epichloë fungal endophytes play a fundamental role in New Zealand grasslands. J. R. Soc. N. Z. 2020, 50, 279–298.
- Caradus, J.; Chapman, D.; Cookson, T.; Cotching, B.; Deighton, M.; Donnelly, L.; Ferguson, J.; Finch, S.; Gard, S.; Hume, D. Epichloë endophytes–new perspectives on a key ingredient for resilient perennial grass pastures. NZGA Res. Pract. Ser. 2021, 17, 57–70.
- Fletcher, L.R.; Markham, L.J.; White, S.R. Endophytes and heat tolerance in lambs grazing perennial ryegrass. N. Z. Grassl. Assoc. 1994, 56, 265–270.
- Popay, A.J.; Hume, D.E. Endophytes improve ryegrass persistence by controlling insects. NZGA Res. Pract. Ser. 2011, 15, 149–156.
- Tapper, B.A.; Lane, G.A. Janthitrems found in a Neotyphodium endophyte of perennial ryegrass. In Proceedings of the 5th International Symposium on Neotyphodium/Grass Interactions, Fayetteville, AR, USA, 23–26 May 2004; p. Poster 301.
- Stewart, A.V.; Kerr, G.A.; Lissaman, W.; Rowarth, J.S. Endophyte in ryegrass and fescue. In Pasture and Forage Plants for New Zealand; Grassland Research and Practice Series 8; Stewart, A.V., Kerr, G.A., Lissaman, W., Rowarth, J.S., Eds.; New Zealand Grassland Association: Mosgiel, New Zealand, 2014; Chapter 8; pp. 66–77.
- Logan, C.M.; Edwards, G.; Kerr, G.; Williams, S. Ryegrass Staggers and Liveweight Gain of Ewe Lambs and Hoggets Grazing Four Combinations of Perennial Ryegrass and Strains of Endophyte. New Zealand Society of Animal Production, 2015. Available online: https://researcharchive.lincoln.ac.nz/handle/10182/9991 (accessed on 24 August 2021).
- Eady, C.; Corkran, J.; Bailey, K.; Kerr, G.; Nicol, A. Estimation of ergovaline intake of cows from grazed perennial ryegrass containing NEA2 or standard endophyte. J. N. Z. Grassl. 2017, 79, 197–203.
- Fletcher, L.; Finch, S.; Sutherland, B.; de Nicolo, G.; Mace, W.; Van Koten, C.; Hume, D. The occurrence of ryegrass staggers and heat stress in sheep grazing ryegrass-endophyte associations with diverse alkaloid profiles. N. Z. Vet. J. 2017, 65, 232–241.
- Hewitt, K.G.; Mace, W.J.; McKenzie, C.M.; Matthew, C.; Popay, A.J. Fungal alkaloid occurrence in endophyte-infected perennial ryegrass during seedling establishment. J. Chem. Ecol. 2020, 46, 410–421.
- Popay, A.J.; Hume, D.E.; Mace, W.J.; Faville, M.J.; Finch, S.C.; Cave, V.M. A root aphid, Aploneura lentisci is affected by Epichloë endophyte strain and impacts perennial ryegrass growth in the field. Crop Pasture Sci. 2021, 72, 155–164.
- Barker, G.M.; Patchett, B.J.; Cameron, N.E. Epichloë uncinata infection and loline content afford Festulolium grasses protection from black beetle (Heteronychus arator). N. Z. J. Agric. Res. 2015, 58, 35–56.
- Barker, G.; Patchett, B.; Gillanders, T.; Brown, G.; Montel, S.; Cameron, N. Feeding and oviposition by Argentine stem weevil on Epichloe uncinata-infected loline-containing Festulolium. N. Z. Plant Prot. 2015, 68, 212–217.
- Fletcher, L.R.; Fletcher, C.G.; Sutherland, B.L. The health and performance of sheep grazing a non-toxic tall fescue endophyte association. In Proceedings of the Fourth International Neotyphodium/ Grass Interactions Symposium, Soest, Germany, 27–29 September 2000; pp. 459–464.
- Popay, A.J.; Tapper, B.A. Endophyte effects on consumption of seed and germinated seedlings of ryegrass and fescue by grass grub (Costelytra zealandica) larvae. In Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses, Christchurch, New Zealand, 25–28 March 2007; pp. 353–356.
- Beck, P.A.; Stewart, C.B.; Gunter, S.A.; Singh, D. Evaluation of tall fescue for stocker cattle in the Gulf coastal plain. Prof. Anim. Sci. 2009, 25, 569–579.
- ACIL Allen Consulting. New Zealand’s Science System: Case Studies, Report to the Ministry of Business, Innovation and Employment. 2017. Available online: https://www.csiro.au/-/media/About/Files/CSIRO-Value-Final-Report-2017-PDF.pdf (accessed on 24 August 2021).
- Duckett, S.K.; Andrae, J.G.; Bouton, J.H.; Hoveland, C.S.; McCann, M.A. Subsequent feedlot performance and carcass quality of steers that grazed tall fescue with different endophyte types. Crop Forage Turfgrass Manag. 2016, 2, 1–7.
- Duckett, S.; Lacy, R.; Andrae, J.; Hoveland, C.; Bouton, J.; McCann, M. Animal performance, carcass quality and economics of cattle finished after grazing endophyte-infected, endophyte-free or nonergot alkaloid-producing endophyte-infected tall fescue. N. Z. Grassl. Assos. Res. Pract. Ser. 2006, 13, 253–255.
- Bouton, J.; Hill, N.S.; Hoveland, C.S.; McCann, M.A.; Thompson, F.N. Performance of tall fescue cultivars infected with non-toxic endophytes. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000; pp. 179–185.
- Finch, S.; Pennell, C.; Kerby, J.; Cave, V. Mice find endophyte-infected seed of tall fescue unpalatable–implications for the aviation industry. Grass Forage Sci. 2016, 71, 659–666.
- Pennell, C.G.; Rolston, M.P. Avanex™ Unique endophyte technology-bird deterrent endophytic grass for amenity turf and airports. In Proceedings of the 22nd International Grasslands Congress, Syndey, Australia, 15–19 September 2013; pp. 405–408.
- Pennell, C.; Rolston, M.; Baird, D.; Hume, D.; McKenzie, C.; Card, S. Using novel-grass endophyte associations as an avian deterrent. N. Z. Plant Prot. 2017, 70, 255–264.
- Pennell, C.; Rolston, M.; Van Koten, C.; Hume, D.; Card, S. Reducing bird numbers at New Zealand airports using a unique endophyte product. N. Z. Plant Prot. 2017, 70, 224–234.
- Pennell, C.G.; Rolston, M.P.; Latham, A.D.M.; Mace, W.J.; Vlaming, B.; van Koten, C.; Latham, M.C.; Brown, S.; Card, S.D. Novel grass–endophyte associations reduce the feeding behaviour of invasive European rabbits (Oryctolagus cuniculus). Wildl. Res. 2017, 43, 681–690.
- Card, S.D.; Faville, M.J.; Simpson, W.R.; Johnson, R.D.; Voisey, C.R.; De Bonth, A.C.M.; Hume, D.E. Mutualistic fungal endophytes in the Triticeae—Survey and description. FEMS Microb. Ecol. 2014, 88, 94–106.
- Simpson, W.R.; Faville, M.J.; Moraga, R.A.; Williams, W.M.; Mcmanus, M.T.; Johnson, R.D. Epichloë fungal endophytes and the formation of synthetic symbioses in Hordeeae (= Triticeae) grasses. J. Syst. Evol. 2014, 52, 794–806.
- Hume, D.E.; Drummond, J.B.; Rolston, M.P.; Simpson, W.R.; Johnson, R.D. Epichloë endophyte improves agronomic performance and grain yield of rye (Secale cereale). In Proceedings of the 10th International Symposium on Fungal Endophytes of Grasses, Salamanca, Spain, 18–21 June 2018; p. 102.
- Simpson, W.R.; Popay, A.J.; Mace, W.J.; Hume, D.E.; Johnson, R.D. Creating synthetic symbioses between Epichloë and rye (Secale cereale) to improve crop performance. In Proceedings of the 10th International Symposium on Fungal Endophytes of Grasses, Salamanca, Spain, 18–21 June 2018; p. 96.
- Popay, A.; Jensen, J.B. Insect protection provided by loline-producing endophytes infecting rye. In Proceedings of the 10th International Symposium on Fungal Endophytes of Grasses, Salamanca, Spain, 18–21 June 2018; p. 103.
- Johnson, R.D.; Tsujimoto, H.; Hume, D.E.; Mace, W.J.; Simpson, W.R. Alien chromatin from Hordeeae grasses enhances the compatibility of Epichloë endophyte symbiosis with the hexaploid wheat Triticum aestivum. In Proceedings of the 10th International Symposium on Fungal Endophytes of Grasses, Salamanca, Spain, 18–21 June 2018; p. 104.
- Johnson, L.J.; Caradus, J.R. The science required to deliver Epichloë endophytes to commerce. In Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019; pp. 343–370.
- Johnson, L.J.; Bastías, D.A.; Caradus, J.R.; Chettri, P.; Forester, N.T.; Mace, W.J.; Miller, T.A.; Moon, C.D.; Voisey, C.R.; Zhang, W. The dynamic mechanisms underpinning symbiotic Epichloë–grass interactions: Implications for sustainable and resilient agriculture. In Microbiome Stimulants for Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 73–108.
