Lactoferrins are an iron-binding glycoprotein that have important protective roles in the mammalian body through their numerous functions, which include antimicrobial, antitumor, anti-inflammatory, immunomodulatory, and antioxidant activities. Among these, their antimicrobial activity has been the most studied, although the mechanism behind antimicrobial activities remains to be elucidated.
- lactoferrin
- lactoferricin
- peptides
- antimicrobial activity
- mechanisms of action
1. Introduction
Lactoferrins are iron-binding proteins that belong to the transferrin family. Since the first isolation of lactoferrins from both bovine [1] and human [2][3] milk in 1960, they have been the subject of intensive structural and functional studies, especially because of their numerous functions, properties, and applications in the food and pharmaceutical industries. Lactoferrins have also been identified in other mammalian species, as listed in Table 1 ; however, bovine and human lactoferrins have been the most studied to date.
| Order | Species | Source of Lactoferrin Isolation | Reference |
|---|---|---|---|
| Primates | Human | Colostrum, milk, tears, nasal/bronchial secretions, saliva, bile/pancreatic secretions (i.e., gastric/intestinal fluids), urine, seminal/vaginal fluids, granules of neutrophils | [4][5][6][7] |
| Rhesus monkey | Milk | [8] | |
| Patas monkey, macaque, baboon, orangutan | Granules of neutrophils | [6] | |
| Carnivores | Dog, bear, domestic cat, tiger, jaguar, cougar, meerkat, otter, tayra, palm civet | Granules of neutrophils | [9][6] |
| Rodents | Rat, hamster, aguti | Granules of neutrophils | [6] |
| Mouse, guinea pig | Milk, granules of neutrophils | [4][6] | |
| Lagomorpha | Rabbit | Granules of neutrophils | [6][10] |
| Artiodactyla | Sheep, buffalo, alpaca, camel | Milk | [7][11][12] |
| Deer | Granules of neutrophils | [4][6] | |
| Cow, goat, pig | Milk, granules of neutrophils | [6] | |
| Perissodactyla | Horse | Milk, granules of neutrophils | [4][6] |
| Proboscidea | Elephant (Asian and African) | Milk | [7][13] |
| Didelphimorphia | Opossum | Granules of neutrophils | [6] |
| Cingulata | Armadillo | Granules of neutrophils | [6] |
Numerous functions have been attributed to lactoferrins, which have ranged from antimicrobial (i.e., antibacterial, antivirus, antifungal, and antiparasitic) to antitumor, anti-inflammatory, immunomodulatory, and antioxidant activities.
Antibacterial activity was the first of these to be ascribed to lactoferrins, which have been well studied [14][15][16], since they represent a great potential as a natural defense agent. Lactoferrin and lactoferrin-derived peptides not only have a broad specter of antibacterial activities against Gram-positive and Gram-negative bacteria and can be potentially used as natural antibiotic in human and veterinary medicine [14] but also have a broad spectrum of activities against enveloped and naked viruses [17][18][19], fungi, yeast [20][21], and parasites [21]. Furthermore, lactoferrin has also been proposed to be potent as a treatment drug in the current COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2) [22][23][24][25][26]. The antimicrobial activity of lactoferrin is related to its ability to chelate iron and thus to deprive microorganisms of these important nutrient, although lactoferrin also expresses antimicrobial activity in the iron-independent pathway by direct interaction with microorganisms.
As lactoferrins were shown to be in body fluids that usually interact with the surrounding environment and considering their broad activity against different microbes, it is initially believed that lactoferrins have an important role in the initiation of the immune system.
2. Antibacterial Activities of Lactoferrins and Lactoferrin-Derived Peptides
Another aspect of the lactoferrin antimicrobial activity might arise from the binding of bovine lactoferrin to porins. Porins are transmembrane proteins that form channels for nonspecific diffusion of hydrophilic solutes across the outer membrane of Gram-negative bacteria [27]. Binding of bovine lactoferrin to porins OmpF and OmpC has been demonstrated [28][29].
Arnold et al. (1982) showed that the preincubation of S. mutans with human lactoferrin reduced glucose uptake and inhibited the synthesis of lactic acid, which indicated that lactoferrins can also affect glucose metabolism [30]. Furthermore, synergistic actions of lactoferrins with lysozymes (the major enzymatic components in the granules of polymorphonuclear lymphocytes) [31], bacteriophages [32], and antibiotics against different bacteria have been demonstrated [33]. By releasing LPS from the Gram-negative bacteria outer membrane, lactoferrins increase the permeability of the outer bacterial membrane and the susceptibility of Gram-negative bacteria to lysozymes, which have an important role in mammal defense mechanisms [31].
For human lactoferricin, the number of amino acids and the structure originally reported by Bellamy et al. (1992a) indicated 47 amino acids (1–47 of human lactoferrin) and included a region homologous to bovine lactoferricin. The sequence of human lactoferricin was reported as two subfragments connected by disulfide bonds between cysteine residues: the linear residues 1 to 11 and the cyclic residues 12 to 47 ( Figure 1 C) [34]. However, Hunter et al. (2005) later reported human lactoferricin as a 49-amino-acid peptide. They also proposed different human lactoferricin structures that are cyclic, although with a continuous polypeptide chain ( Figure 1 D) [35].
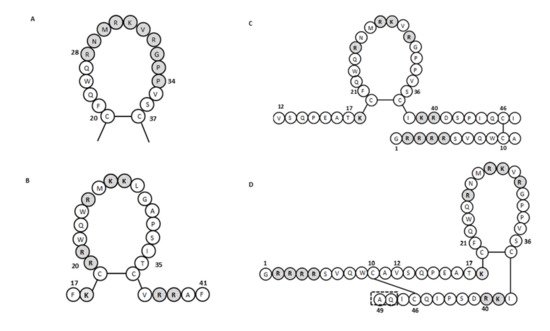
To determine the active regions of bovine and human lactoferricins, several synthetic peptides were made for each. All bovine lactoferricins inhibited bacterial growth; however, only to modified human lactoferricins showed activities against the bacteria tested, while non-modified peptides that corresponding to different regions of native human lactoferricin showed no antimicrobial activities [37]. In contrast, the small 11-amino-acid peptide (21–31 of human lactoferrin) that is homologous to the helical and loop regions of human lactoferrin and lactoferricin, respectively, inhibited growth of E. coli and bound LPS [38]. This thus indicated that this region has an important role in the antibacterial activity of human lactoferrin and lactoferricin.
3. Antifungal Activities of Lactoferrins and Lactoferrin-Derived Peptides
Antifungal activity for human lactoferrin was first reported by Kirkpatrick et al. (1971), where inhibition of growth was demonstrated against the yeast Candida albicans [39]. Human lactoferrin also inhibited the growth of Candida krusei , to a greater extent than seen for C. albicans . The inhibition of each of these was dose dependent [40][41], while iron saturation resulted in the loss of this antifungal activity of human lactoferrin [39][40][41]. Soon after the first isolation of bovine lactoferricin, its antifungal activity was extensively studied ( Supplementary Table S1 ), including for fungi that cause dermatophytosis. Using [14C]-labeled bovine lactoferricin, its direct binding was shown [42], along with its potent disruptive effects on C. albicans cell membranes [43]. Furthermore, for pathogenic fungi, bovine lactoferricin inhibited the uptake of [3H]-glucose in Trichophyton rubrum and caused substantial changes to the ultrastructure of Trichophyton mentagrophytes , which included dense aggregation of the cytoplasmic materials [44]. The anti- Candida activity of bovine and human lactoferricins can be affected by different pHs, temperatures, and ions (i.e., phosphate, bicarbonate, Ca 2+ , and Mg 2+ ) [41][42]. Indeed, bovine lactoferricin binding to C. albicans was reduced by the addition of the divalent cations Ca 2+ and Mg 2+ ,and was pH dependent, as also seen for its antimicrobial activity [42].
To further determine the antifungal activities of bovine lactoferricin, several lactoferricin-like peptides were tested by Ueta et al. (2001), using synthetic bovine lactoferricin and four of its derived peptides. Among these peptides, peptide 2 (amino acids 17–26) showed the greatest suppression of multiplication of Candida cells, while the other peptides showed only weak activities [45]. Munoz and Marcos (2006) tested two bovine lactoferricin-derived peptides, lactoferricin 17–31 and lactoferricin 20–25, against different bacteria and fungi that are causative agents for plant diseases [46], while van der Kraan et al. (2004) tested the anti- Candida activities of bovine lactoferricin fragment 17–30 [47]. These data showed that bovine lactoferricin (20–25) was less active than the more extended bovine lactoferricin (17–31), with the exception of Botrytis cinerea , where very similar activities were seen, and that filamentous fungi were more susceptible than bacteria or yeast [46]. Peptide 2 of Ueta et al. (2001) also did not bind iron, which indicated that its mechanism of anti- Candida activity was unrelated to depriving these yeast of this nutrient [45]. Three peptides obtained by tryptic digestion of bovine lactoferrin (i.e., 21LF, 38LF, and 45LF) showed lower antibacterial activities than the native protein; however, their antifungal activities were greater than that of lactoferrin [48]. Furthermore, the human lactoferricin 1–11 synthetic peptide inhibited biofilm formation by C. albicans mainly at the early stages, showing interference with the cell density of the biofilm and the metabolic activity [49].
Candida albicans has also shown high susceptibility to synthetic lactoferrampin 268–284 and other lactoferrampin-like peptides [47][50][51]. To define the antimicrobial region of bovine lactoferrampin, van der Kraan et al. (2005) synthesized a series of lactoferrampin peptides. They concluded that the positively charged amino acids of the C-terminal of lactoferrampin 265–284 were crucial for its candidacidal activity, while the N-terminal part was essential for activity because it facilitated helix formation [51]. Interestingly human lactoferrampin showed no inhibitory effects against C. albicans unless a lysine residue was added to the C-terminus of molecule or a negatively charged aspartic acid was mutated to asparagine [52].
4. Antiparasitic Activities of Lactoferrins and Lactoferrin-Derived Peptides
Lactoferrin and lactoferrin-derived peptides also exert antiparasitic activity against different protozoa and small parasites. Lactoferrin inhibited the in vitro growth of Babesia caballi and Babesia equi ; however, the inhibitory effect was greater for B. caballi and only occurred in the presence of apo form of lactoferrin [53]. Since many microorganisms, including parasites, require iron for growth and development, iron binding proteins lactoferrin can contribute to host defense against parasites by sequestrating this important nutrient from microorganisms [54]. Furthermore, human and bovine lactoferrin ant lactoferrin-derived peptides (including lactoferricin, lactoferampin, and Lf-chimera) also showed antiparasitic activity against Giardia lamblia [55][56] and Giardia intestinalis [57]. The most gardicidal effect was observed for bovine lactoferrin-derived peptides (50% lethal dose (LD 50s ) of 8 µg/mL) followed by human-derived peptides, bovine lactoferrin (LD 50s of 1.2 mg/mL), and human lactoferrin (LD 50s of 1.5 mg/mL), indicating that bovine lactoferrin is more potent that human lactoferrin [55]. Furthermore, it has been shown that lactoferrins and lactoferrin-derived peptides bind on the surface of G. lamblia [56][58]. The gardicidal effect of lactoferrin and lactoferrin-derived peptides was also observed at low concentrations, where they caused dilation of the endoplasmic reticulum (ER) membranes, expansion of the nuclear membrane, and plasma membrane protrusions [56], although high concentrations cause severe morphological changes or even induce programmed cell death [57][58]. Lactoferrin also exhibited antiparasitic activity against Cryptosporidium parvum sporozoites but had no significant effect on oocysts viability or parasite intracellular development [59].
Some protozoan parasites such as Trichomonas, Giardia, and Entamoeba require a high extracellular iron concentration for their growth [60][61] and have therefore adapted to acquire extracellular iron from other sources such as host iron-binding proteins such as transferrin and lactoferin. It is well known that Trypanosoma brucei can sequestrate iron from transferrin by binding through a surface receptor [62]. Iron uptake from transferrin and lactoferrin has also been demonstrated for Trichomonas vaginalis [61] , Trichomonas foetus [63], and Leishmania chagasi [64]; however, in the last case, other possible mechanism of iron sequestrating have been proposed [65]. Binding sites for lactoferrin has also been demonstrated in T. brucei [66] and Toxoplasma gondii [67][68][69]; however, in T. gondii, binding sites were specific for lactoferrin since the absence of transferrin binding was observed [69]. It is possible that protozoa binding sites for lactoferrin could have roles in iron acquisition; however, this is yet unclear since iron saturation had no impact on binding pattern [66][67]. Furthermore, studies by Tanaka et al. and Dzitoko et al. showed that lactoferrins did not prevent parasite penetration into host cell or had direct cytotoxic impact on T. gondii viability [70][71][72]; however, the inhibition of protozoa multiplication by lactoferrin was demonstrated [72]. Furthermore, when the lactoferrin-derived peptide lactoferricin was applied, a reduced viability, cyst formation in mouse brains, infectivity of sporozoites, and decreased penetration activity by T. gondii was observed [70][73][74]. Reduced infectivity of sporozoites was also observed in a case of Eimeria stiedai [74].
This entry is adapted from the peer-reviewed paper 10.3390/ijms222011264
References
- Groves, M.L. The isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350.
- Johansson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512.
- Montreuil, J.; Tonnelat, J.; Mullet, S. Preparation and properties of lactosiderophilin (lactotransferrin) of human milk. Biochim. Biophys. Acta 1960, 45, 413–421.
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B 1971, 39B, 119–129.
- Masson, P.L.; Heremans, J.F.; Dive, C.H. An iron-binding protein common to many external secretions. Clin. Chim. Acta 1966, 14, 735–739.
- Barton, J.C.; Parmley, R.T.; Butler, T.W.; Williamson, S.; Mackenzie, S.; Chandler, D.B.; Blackburn, W.; Heck, L.W., Jr. Neutrophil lactoferrin content: Variation among mammals. Anat. Rec. 1988, 221, 567–575.
- Conesa, C.; Sánchez, L.; Rota, C.; Pérez, M.D.; Calvo, M.; Farnaud, S.; Evans, R.W. Isolation of lactoferrin from milk of different species: Calorimetric and antimicrobial studies. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 131–139.
- Davidson, L.A.; Lönnerdal, B. Isolation and characterization of Rhesus monkey milk actoferrin. Pediatr. Res. 1986, 20, 197–201.
- Berlov, M.N.; Korableva, E.S.; Andreeva, Y.V.; Ovchinnikova, T.V.; Kokryakov, V.N. Lactoferrin from canine neutrophils: Isolation and physicochemical and antimicrobial properties. Biochemistry 2007, 72, 445–451.
- Baggiolini, M.; de Duve, C.; Masson, P.L.; Heremans, J.F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J. Exp. Med. 1970, 131, 559–570.
- Elagamy, E.I.; Ruppanne, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Purification and Characterization of lactoferrin; lactoperoxidase; lysozyme and immunoglobulins from Camel’s Milk. Int. Dairy J. 1996, 6, 129–145.
- Qian, Z.Y.; Jollès, P.; Migliore Samour, D.; Fiat, A.M. Isolation and characterization of sheep lactoferrin; an inhibitor of platelet aggregation and comparison with human lactoferrin. Biochim. Biophys. Acta 1995, 1243, 25–32.
- Stumpf, P.; Welch, U. Secretory and defensive functions of the duct system of the lactating mammary gland of the African elephant (Loxodonta africana; Proboscidea). Zoomorphology 2004, 123, 155–167.
- Bruni, N.; Capucchio, M.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752.
- Jenssen, H.; Hancock, R. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29.
- Zarzosa Moreno, D.; Avalos Gómez, C.; Ramírez Texcalco, L.; Torres López, E.; Ramírez Mondragón, R.; Hernández Ramírez, J.; Serrano Luna, J.; de la Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763.
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018.
- Redwan, E.; Uversky, V.; El Fakharany, E.; Al Mehdar, H. Potential lactoferrin activity against pathogenic viruses. CR Biol. 2014, 337, 581–595.
- Seganti, L.; Di Biase, A.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral activity of lactoferrin towards naked viruses. Biometals 2004, 17, 295–299.
- Fernandes, K.; Carter, D. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2.
- Leboffe, L.; Giansanti, F.; Antonini, G. Antifungal and Antiparasitic Activities of Lactoferrin. Anti Infect. Agents Med. Chem. 2009, 8, 114–127.
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903.
- Chang, R.; Ng, T.; Sun, W. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118.
- Elnagdy, S.; AlKhazindar, M. The Potential of Antimicrobial Peptides as an Antiviral Therapy against COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 780–782.
- Wang, Y.; Wang, P.; Wang, H.; Luo, Y.; Wan, L.; Jiang, M.; Chu, Y. Lactoferrin for the treatment of COVID-19 (Review). Exp. Ther. Med. 2020, 20, 272.
- Zimecki, M.; Actor, J.; Kruzel, M. The potential for Lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 2021, 95, 107571.
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656.
- Erdei, J.; Forsgren, A.; Naidu, A.S. Lactoferrin Binds to Porins OmpF and OmpC in Escherichia coli. Infect. Immun. 1994, 62, 1236–1240.
- Naidu, S.S.; Svensson, U.; Kishore, A.R.; Naidu, A.S. Relationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. Antimicrob. Agents Chemother. 1993, 37, 240–245.
- Arnold, R.R.; Russell, J.E.; Champion, W.J.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Differentiation from the stasis of iron deprivation. Infect. Immun. 1982, 35, 792–799.
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091.
- Zimecki, M.; Artym, J.; Kocieba, M.; Weber Dabrowska, B.; Lusiak Szelachowska, M.; Górski, A. The concerted action of lactoferrin and bacteriophages in the clearance of bacteria in sublethally infected mice. Postepy. High Med. Dosw. 2008, 62, 42–46.
- Al Mogbel, M.; Menezes, G.; Elabbasy, M.; Alkhulaifi, M.; Hossain, A.; Khan, M. Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens. Medicina 2021, 57, 343.
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136.
- Hunter, H.N.; Demcoe, A.R.; Jenssen, H.; Gutteberg, T.J.; Vogel, H.J. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob. Agents Chemother. 2005, 49, 3387–3395.
- Elass Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28–34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845.
- Farnaud, S.; Spiller, C.; Moriarty, L.C.; Patel, A.; Gant, V.; Odell, E.W.; Evans, R.W. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol. Lett. 2004, 233, 193–199.
- Chapple, D.S.; Hussain, R.; Joannou, C.L.; Hancock, R.E.; Odell, E.; Evans, R.W.; Siligardi, G. Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide. Antimicrob. Agents Chemother. 2004, 48, 2190–2198.
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971, 124, 539–544.
- Nikawa, H.; Samaranayake, L.P.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 1993, 38, 1057–1063.
- Soukka, T.; Tenovuo, J.; Lenander Lumikari, M. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol. Lett. 1992, 90, 223–228.
- Bellamy, W.; Wakabayashi, H.; Takase, M.; Kawase, K.; Shimamura, S.; Tomita, M. Killing of Candida albicans by lactoferricin B; a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993, 182, 97–105.
- Wakabayash, H.; Hiratani, T.; Uchida, K.; Yamaguch, H. Antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin. J. Infect. Chemother. 1996, 1, 185–189.
- Bellamy, W.; Yamauchi, K.; Wakabayashi, H.; Takase, M.; Takakura, N.; Shlmamura, S.; Tomita, M. Antifungal properties of lactoferricin B; a peptide derived from the N-terminal region of bovine lactoferrin. Lett. Appl. Microbiol. 1994, 18, 230–233.
- Ueta, E.; Tanida, T.; Osaki, T. A novel bovine lactoferrin peptide; FKCRRWQWRM; suppresses Candida cell growth and activates neutrophils. J. Pept. Res. 2001, 57, 240–249.
- Munoz, A.; Marcos, J.F. Activity and mode of action against fungal phytopathogens of bovine lactoferricin-derived peptides. J. Appl. Microbiol. 2006, 101, 1199–1207.
- Van der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183.
- Rastogi, N.; Nagpal, N.; Alam, H.; Pandey, S.; Gautam, L.; Sinha, M.; Shin, K.; Manzoor, N.; Virdi, J.S.; Kaur, P.; et al. Preparation and antimicrobial action of three tryptic digested functional molecules of bovine lactoferrin. PLoS ONE 2014, 9, e90011.
- Morici, P.; Fais, R.; Rizzato, C.; Tavanti, A.; Lupetti, A. Inhibition of Candida albicans biofilm formation by the synthetic lactoferricin derived peptide hLF1-11. PLoS ONE 2016, 11, e0167470.
- Bolscher, J.G.M.; van der Kraan, M.I.A.; Nazmi, K.; Kalay, H.; Gru, C.H.; vant Hof, W.; Veerman, E.C.I.; Nieuw Amerongen, A.V. A one-enzyme strategy to release an antimicrobial peptide from the LFampin-domain of bovine lactoferrin. Peptides 2006, 27, 1–9.
- van der Kraan, M.I.; Nazmi, K.; Teeken, A.; Groenink, J.; van’t Hof, W.; Veerman, E.C.; Bolscher, J.G.; Nieuw Amerongen, A.V. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin; exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2005, 386, 137–142.
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.M.; Vogel, H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 2009, 91, 141–154.
- Ikadai, H.; Tanaka, T.; Shibahara, N.; Tanaka, H.; Matsuu, A.; Kudo, N.; Shimazaki, K.; Igarashi, I.; Oyamada, T. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712.
- Wilson, M.; Britigan, B. Iron acquisition by parasitic protozoa. Parasitol. Today 1998, 14, 348–353.
- Turchany, J.; Aley, S.; Gillin, F. Giardicidal activity of lactoferrin and N-terminal peptides. Infect. Immun. 1995, 63, 4550–4552.
- Frontera, L.; Moyano, S.; Quassollo, G.; Lanfredi Rangel, A.; Rópolo, A.; Touz, M. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018, 1–15.
- Aguilar Diaz, H.; Canizalez Roman, A.; Nepomuceno Mejia, T.; Gallardo Vera, F.; Hornelas Orozco, Y.; Nazmi, K.; Bolscher, J.; Carrero, J.; Leon Sicairos, C.; Leon Sicairos, N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017, 95, 82–90.
- Turchany, J.; McCaffery, J.; Aley, S.; Gillin, F. Ultrastructural effects of lactoferrin binding on Giardia lamblia trophozoites. J. Eukaryot. Microbiol. 1997, 44, 68–72.
- Paredes, J.; Sparks, H.; White, A., Jr.; Martinez Traverso, G.; Ochoa, T.; Castellanos González, A. Killing of Cryptosporidium sporozoites by Lactoferrin. Am. J. Trop. Med. Hyg. 2017, 97, 774–776.
- Weinberg, E. Iron and Susceptibility to Infectious Disease. Science 1974, 184, 952–956.
- Sehgal, R.; Goyal, K.; Sehgal, A. Trichomoniasis and lactoferrin: Future prospects. Infect. Dis. Obstet. Gynecol. 2012, 1–8.
- Kariuki, C.; Stijlemans, B.; Magez, S. The Trypanosomal Transferrin Receptor of Trypanosoma Brucei—A Review. Trop. Med. Infect. Dis. 2019, 4, 126.
- Tachezy, J.; Kulda, J.; Bahníková, I.; Suchan, P.; Rázga, J.; Schrével, J. Tritrichomonas foetus: Iron acquisition from lactoferrin and transferrin. Exp. Parasitol. 1996, 83, 216–228.
- Wilson, M.; Vorhies, R.; Andersen, K.; Britigan, B. Acquisition of iron from transferrin and lactoferrin by the protozoan Leishmania chagasi. Infect. Immun. 1994, 62, 3262–3269.
- Wilson, M.; Lewis, T.; Miller, M.; McCormick, M.; Britigan, B. Leishmania chagasi: Uptake of iron bound to lactoferrin or transferrin requires an iron reductase. Exp. Parasitol. 2002, 100, 196–207.
- Tanaka, T.; Abe, Y.; Inoue, N.; Kim, W.; Kumura, H.; Nagasawa, H.; Igarashi, I.; Shimazaki, K. The detection of bovine lactoferrin binding protein on Trypanosoma brucei. J. Vet. Med. Sci. 2004, 66, 619–625.
- Tanaka, T.; Abe, Y.; Kim, W.; Xuan, X.; Nagasawa, H.; Igarashi, I.; Kumura, H.; Shimazaki, K. The detection of bovine lactoferrin binding protein on Toxoplasma gondii. J. Vet. Med. Sci. 2003, 65, 1377–1380.
- Dziadek, B.; Dziadek, J.; Dlugonska, H. Identification of Toxoplasma gondii proteins binding human lactoferrin: A new aspect of rhoptry proteins function. Exp. Parasitol. 2007, 115, 277–282.
- Dziadek, B.; Dzitko, K.; Dlugonska, H. Toxoplasma gondii binds human lactoferrin but not transferrin. Exp. Parasitol. 2005, 110, 165–167.
- Tanaka, T.; Omata, Y.; Saito, A.; Shimazaki, K.; Yamauchi, K.; Takase, M.; Kawase, K.; Igarashi, K.; Suzuki, N. Toxoplasma gondii: Parasiticidal effects of bovine lactoferricin against parasites. Exp. Parasitol. 1995, 81, 614–617.
- Tanak, T.; Omata, Y.; Saito, A.; Shimazaki, K.; Igarashi, I.; Suzuki, N. Growth Inhibitory Effects of Bovine Lactoferrin to Toxoplasma gondii Parasites in Murine Somatic Cells. J. Vet. Med. Sci. 1996, 58, 61–65.
- Dzitko, K.; Dziadek, B.; Dziadek, J.; Długońska, H. Toxoplasma gondii: Inhibition of the Intracellular Growth by Human Lactoferrin. Pol. J. Microbiol. 2007, 56, 25–32.
- Isamida, T.; Tanaka, T.; Omata, Y.; Yamauchi, K.; Shimazaki, K.; Saito, A. Protective effect of lactoferricin against Toxoplasma gondii infection in mice. J. Vet. Med. Sci. 1998, 60, 241–244.
- Omata, Y.; Satake, M.; Maeda, R.; Saito, A.; Shimazaki, K.; Yamauchi, K.; Uzuka, Y.; Tanabe, S.; Sarashina, T.; Mikami, T. Reduction of the infectivity of Toxoplasma gondii and Eimeria stiedai sporozoites by treatment with bovine lactoferricin. J. Vet. Med. Sci. 2001, 63, 187–190.
