Malaria is a life-threatening global epidemic disease and has caused more than 400,000 deaths in 2019. To control and prevent malaria, the development of a vaccine is a potential method. An effective malaria vaccine should either combine antigens from all stages of the malaria parasite’s life cycle, or epitopes of multiple key antigens due to the complexity of the Plasmodium parasite. Malaria’s random constructed antigen-1 (M.RCAg-1) is one of the recombinant vaccines, which was selected from a DNA library containing thousands of diverse multi-epitope chimeric antigen genes. Moreover, besides selecting an antigen, using an adjuvant is another important procedure for most vaccine development procedures. Freund’s adjuvant is considered an effective vaccine adjuvant for malaria vaccine, but it cannot be used in clinical settings because of its serious side effects. Traditional adjuvants, such as alum adjuvant, are limited by their unsatisfactory immune effects in malaria vaccines, hence there is an urgent need to develop a novel, safe and efficient adjuvant. In recent years, Pickering emulsions have attracted increasing attention as novel adjuvant. In contrast to classical emulsions, Pickering emulsions are stabilized by solid particles instead of surfactant, having pliability and lateral mobility. In this study, we selected aluminum hydroxide gel (termed as “alum”) as a stabilizer to prepare alum-stabilized Pickering emulsions (ALPE) as a malaria vaccine adjuvant. In addition, monophosphoryl lipid A (MPLA) as an immunostimulant was incorporated into the Pickering emulsion (ALMPE) to further enhance the immune response. In vitro tests showed that, compared with alum, ALPE and ALMPE showed higher antigen load rates and could be effectively endocytosed by J774a.1 cells. In vivo studies indicated that ALMPE could induce as high antibody titers as Freund’s adjuvant. The biocompatibility study also proved ALMPE with excellent biocompatibility. These results suggest that ALMPE is a potential adjuvant for a malaria vaccine.
1. Introduction
Malaria is an infectious disease caused by an intraerythrocytic protozoa of the genus Plasmodium [
1,
2,
3]. Despite the many efforts that have been made in preventing malaria over the past decade, this disease remains a substantial global health problem. It is a challenge to develop safe and effective vaccines against the spread of malaria. RTS,S is comprised of AS01 and hepatitis B virus surface antigen (HBsAg) virus-like particles incorporating a portion of the Plasmodium falciparum-derived circumsporozoite protein (CSP) genetically fused to HBsAg, which have proven to have bactericidal immunity in mice [
4,
5]. RTS,S, administered with AS01, is a liposome-based vaccine adjuvant containing monophosphorylate lipid A (MPLA) and is a recombinant antigen which was the first malaria vaccine to undergo pilot implementation, and currently vaccinates 360,000 children per year in Malawi, Ghana and Kenya [
6,
7]. However, clinical results have shown that the efficacy of RTS,S was ranging from 26% to 50% in infants and young children [
8]. Therefore, research on malaria vaccines is continuing. when the malaria vaccine is used alone, due to the complex life cycle of the malaria parasite, it cannot trigger a strong immune response, so an adjuvant is needed to assist it [
9]. The combined multi-epitope vaccine is composed of multiple epitopes and can stimulate the body to produce different antigens against the same life stage of Plasmodium, or against different antigens in different life stages, thereby reducing the probability of infection [
10]. The intra-erythrocyte stage is the only stage of Plasmodium falciparum’s pathogenicity and disease. The expression system of M.RCAg-1 is BL21(DE3)-M.RCAg-1/pDS-ex-Ekase, including 4 Cys residues. Eight of the 11 epitope peptides are in the intra-erythrocyte stage, so the antibodies produced in animal experiments can inhibit the growth of Plasmodium falciparum [
11].
2. Results and Discussion
2.1. Pickering Emulsions by Using Alum as Stabilizer
Pickering emulsions are composed of oil phase, particles and aqueous phase. Unlike traditional emulsions, particles in Pickering emulsions are positioned at the interface between oil and water and act as stabilizer to improve droplet stability. In order to make the emulsion formulation suitable for adjuvant application, the Pickering emulsions were prepared using FDA-approved squalene and alum as the oil phase and stable particles respectively.
2.1.1. Effect Particle Concentration on Pickering Emulsions
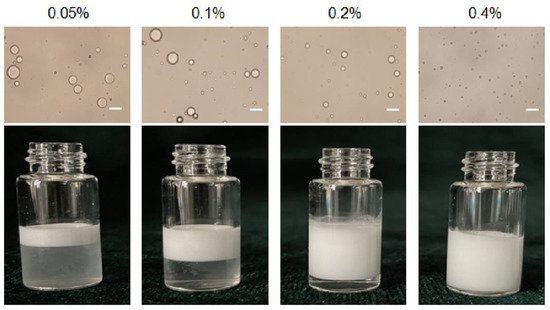
Pickering emulsions with different particle concentrations from 0.05 to 0.4% (
w/
v) and same oil/water ratio 1/9 were prepared as shown in
Figure 2. The emulsion is stratified when the particle concentration is 0.05%, 0.1% and 0.2%, respectively, and the corresponding light microscope images also show that the droplet sizes were inhomogeneous. The size of the emulsion droplets decreased when the particle concentration increased (18 μm to 2.3 μm). The emulsions stabilized by particles with 0.4% concentrations were more stable, because high number of particles covered the droplets more completely. Furthermore, particles might also form networks in the continuous phase surrounding the drops, and hinder coalescence between droplets [
24]. Moreover, the droplet sizes became smaller when particle concentrations increased from 0.05% to 0.4%. This is because more particles were progressively available to stabilize the emulsion, and the droplet size decreased to provide high surface area and accommodate more particles at the interface [
25]. Therefore, 0.4% (
w/
v) serves as the optimal particle concentration to form stable Pickering emulsions.
Figure 2. Optical micrographs and the appearance of Pickering emulsion prepared with different particle concentration. Scale bar = 10 μm.
2.1.2. Effect the Buffer Type of the Aqueous Phase on Emulsions
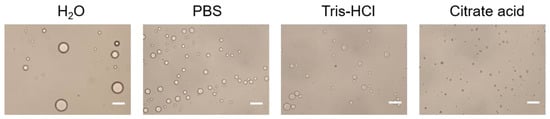
The effect of the buffer type of the aqueous phase on Pickering emulsions was shown in Figure 3. It is illustrated that the droplet size obtained by using H2O as aqueous phase was the largest, and the droplet size obtained by using PBS and Tris-HCl as aqueous phase was inhomogeneous. Compared with other buffer solutions, citrate acid buffer solution for stabilizing Pickering emulsions can obtain particles with uniform droplet size and good dispersibility. The results indicated that citrate acid buffer solution might be the optimal buffer for the aqueous phase. The stability of emulsions might be due to the citrate acid is prone to complexation with alum ions, which forms particles aggregation at the oil-water interface and makes the system stable. Therefore, citrate acid buffer solution is chosen as the aqueous phase.
Figure 3. Optical micrograph of Pickering emulsions prepared with different buffer type of the aqueous phase. Scale bar = 10 μm.
2.1.3. Effect the pH of the Aqueous Phase on Emulsion
Alum particle dispersion (0.4%
w/
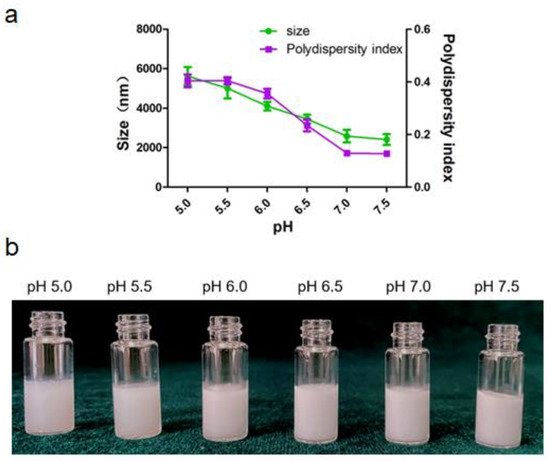
v) was prepared using citrate acid buffers with different pH (5.0–7.5) and sheared with squalene to fabricate Pickering emulsions. The size and the appearance of emulsions varies with aqueous pH were depicted in
Figure 4. The particle surface hydrophobicity can be adjusted by controlling the pH of the aqueous phase, which further affects the wettability of the particle surface and changes its adsorption characteristics at the oil/water interface. As shown in
Figure 4a, the droplet sizes decreased when pH increased. Evidently, the polydispersity index of Pickering emulsion was less than 0.2 when the pH of aqueous phase was above 7.0, which indicated that the Pickering emulsions have uniform size distribution and good dispersibility. All the emulsions presented poor uniformity at pH from 5.0 to 6.0. These observations could be attributed to the decrease in electrostatic repulsions as the pH values increase that led to the collapse or aggregation of polymer chains at the oil-water interface. Correspondingly, the decrease in the repulsion forces might have allowed the deposition of non-adsorbed particles on the droplets, which possibly reinforced the stability of the Pickering emulsions [
26]. Thus, pH of 7.5 was choose as the optimal pH condition for the aqueous phase.
Figure 4. Pickering emulsion varies with pH of the aqueous phase. (a) Size and size distribution of the droplet. (b) The appearance of the emulsion.
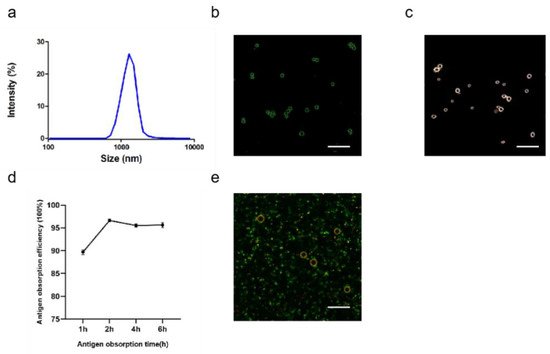
2.1.4. Characterization of ALPE and ALMPE
After optimizing the preparation conditions, ALPE and ALMPE were obtained (
Figure 5 and
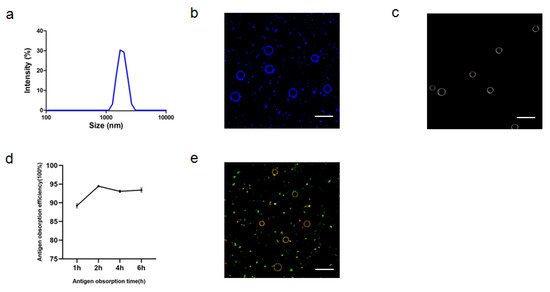
Figure 6). The zeta potentials of ALPE and ALMPE were 19.7 ± 3.2 mV and 21.0 ± 2.9 mV, respectively. The average size of ALPE and ALMPE were 2384 ± 72 nm and 1659 ± 58 nm, the polydispersity index of ALPE and ALMPE were 0.110 ± 0.037 and 0.190 ± 0.056, respectively (
Figure 5a,b). It is speculated that the addition of MLPA makes the particles more adsorbed on the oil-water interface and reduces the surface tension value, thus forming petite droplets. How could objects with such sizes be safely internalized into the cells? Regardless of whether the antigen is desorbed from the surface of the adjuvant, the antigen must enter the lymphatic system before it can reach the lymph nodes, thereby stimulating B cells and T cells. Particles with a size of 20–1000 nm can be taken up by cells (DC or cells that can be swallowed by DC). Particles in the range of 1–30 nm can pass through the lymphatic system for DC internalization or directly enter the lymph nodes as free antigens [
27,
28].
Figure 5. Characteristics of ALPE for adjuvant application. (a) Size distribution of ALPE. (b) Confocal image of ALPE droplets. Alum particles was labeled by lumogallion (blue). (c) Hyperspectral image of ALPE droplets. (d) Antigen adsorption efficiency in different times. (e) Confocal image of antigen-adsorbed ALPE droplets. Alum particles and OVA were labeled by lumogallion (red) and Cy5 (green), respectively. Scale bar = 5 μm. Data are expressed as mean ± s.e.m. (n = 3).
Figure 6. Characteristics of ALMPE for adjuvant application. (a) Size distribution of ALMPE. (b) Confocal image of ALMPE droplets. Alum particles was labeled by lumogallion (green). (c) Hyperspectral image of ALMPE droplets. (d) Antigen obsorption efficiency in different times. (e) Confocal image of antigen-adsorbed ALMPE droplets. Alum particles and OVA were labeled by lumogallion (red) and Cy5 (green), respectively. Scale bar = 5 μm. Data are expressed as mean ± s.e.m. (n = 3).
Due to the rough surface of Pickering emulsions, antigens are absorbed on the surface and cracks of particles. APLE and ALMPE absorbed more than 90% of the antigen in 2 h (Figure 5d,e and Figure 6d,e).
Particulate adjuvants resemble the morphology and size of the native virion displaying a densely repetitive array of epitopes in a limited space, which makes them easily captured and processed by antigen-presenting cells [
29]. Antigens in particulate forms can be efficiently engulfed by dendritic cells and presented to T cells, and these also magnify the priming of T cell as well as B cell response [
30,
31]. In the case of surface-bound antigens, antigenic fluidity can contribute to enhanced antigen-immune cell contact, resulting in efficient endocytosis by antigen presenting cells.
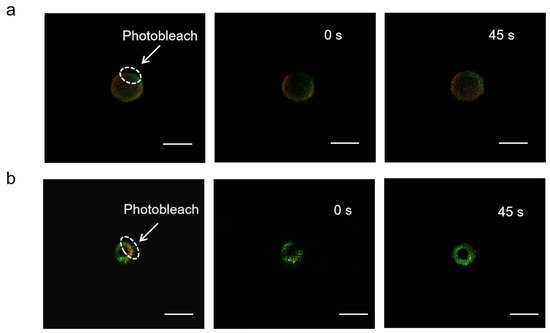
The mobility of adsorbed antigens was demonstrated by fluorescence recovery after photobleaching (FRAP). By adjusting the focal plane to the tangent of the APLE and ALMPE droplets, and quenching the fluorescence of the selected area, monitor the fluorescence recovery of the area in real time. OVA is marked with Cy5 in green and aluminum particles were marked with lumogallion in red. After photobleaching, fluorescence recovery of the particles was not observed, while the fluorescence intensity of OVA was enhanced after 45 s (Figure 7). The results indicated that the antigens demonstrated lateral mobility and might diffuse when in contact with cells, which could dynamically activate the dangerous signal molecules on the surface of immune cells and improved immune response.
Figure 7. FRAP analysis on the lateral mobility of OVA antigens on the surface of ALPE (a) and ALMPE (b) droplets. Alum particles and OVA were labeled by lumogallion (red) and Cy5 (green), respectively. Scale bar = 2 μm.
2.2. Cytosolic Localization
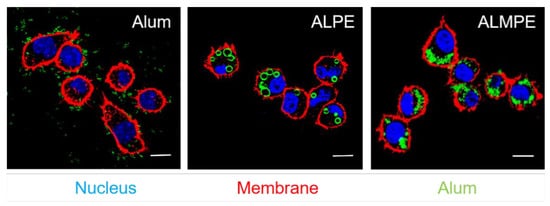
BMDCs were incubated with ALPE to investigate the cellular distribution of ALPE and ALMPE in vitro. As shown in Figure 8, potent cell-residing ALPE and ALMPE were proved, but alum tended to condense on DC membranes without entering the cells. Consequently, ALPE and ALMPE may have the potential to mediate the intracellular transfer of antigens.
Figure 8. Confocal images of endocytosis. DC nucleus were stained by DAPI (blue). DC membrane and alum particles were labeled with TRITC-phalloidin (red) and Lumogallion (green), respectively. Scale bar = 10 μm.
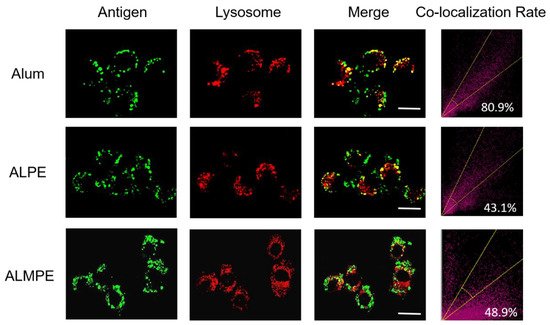
The intracellular distribution of the antigen was then observed with CLSM. For exogenous antigens, efficient endosomal escape is the key step for efficient cytotoxic T lymphocyte response and is essential for enhancing the cellular immune response. Using OVA antigen as model, after incubation 24 h, ALPE and ALMPE obviously increased cytoplasm-residing antigens with lower co-localization compared to Alum (Figure 9). The results indicated that ALPE and ALMPE can promote the uptake of antigen by cells and is expected to provided powerful cellular immune response.
Figure 9. Confocal images of lysosomal escape on BMDCs after 24 h treatment. Lysotracker and OVA antigen were labeled with Lyso Tracker (red) and Cy5 (green). Scale bar = 10 μm.
2.3. Enhanced Systemic Immunity In Vivo (OVA as Model Antigen)
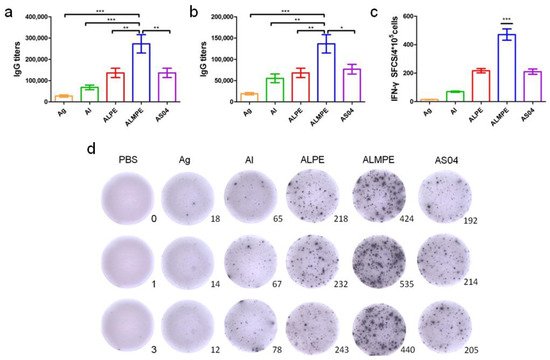
Next, the systemic immune responses in mice after subcutaneous injections were evaluated. For systemic immunity, humoral and cellular immune responses were investigated. As shown in Figure 10a,b, ALPE and AS04 triggered the same level of secretion of specific antibody IgG titters that are higher than alum adjuvants. ALMPE treated mice exhibited strong IgG responses following vaccination, while injection with OVA alone induced only a weak immunogenic response. ALMPE enhanced the production of OVA-specific antibodies by 9.8-fold at day 28 (p < 0.01) and 7.1-fold at day 35 (p < 0.05), as compared with the responses to ALPE and AS04. Then, the impact of ALPE and ALMPE on T cell responses was determined. Furthermore, the number of IFN-γ secreting cells were evaluated at the single cell level via ELISPOT assay. According to Figure 10c,d, the number of IFN-γ-secreting cells in the mice immunized with ALMPE were about 2.24-fold higher than that of ALPE and AS04 treated group (p < 0.005). Consequently, these data showed that ALMPE stimulated an effective humoral and cellular immune responses.
Figure 10. Systemic immunity in vivo. Production of OVA antigen-specific antibodies in the serum at day 28 (a) and day 38 (b). ELISPOT assay on IFN-γ spot-forming cells among splenocytes (c,d). “Ag” and “Al” represent the individual antigen group and alum group respectively. Data are expressed as mean ± s.e.m. (n = 6). * p < 0.05, ** p < 0.01, *** p < 0.005.
2.4. Adjuvant Effects of Pickering Emulsions for M.RCAg-1 Vaccine
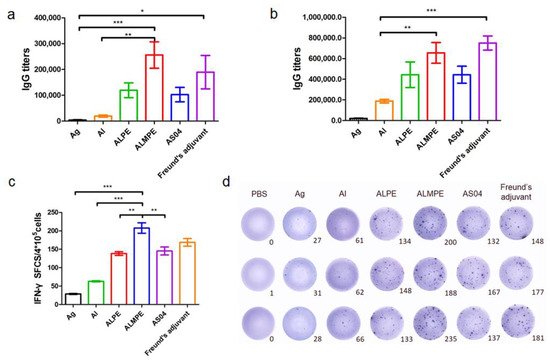
As mentioned above, the adjuvanticity of ALMPE for model antigen OVA was investigated, and it was found that a combination of MPLA significantly improved the adjuvant effects of Pickering emulsions. To further investigate the adjuvant activity, the immune efficacy of Pickering emulsion in M.RCAg-1 vaccinations was evaluated. Firstly, antigen-specific antibody titers in sera were assessed. It is well-known that Freund’s adjuvant possessed a strong adjuvant effect. It’s noteworthy shown in Figure 11a,b, ALMPE induced antibody titers comparable to Freund’s adjuvant, indicating the potent humoral responses. Compared to the M.RCAg-1 antigen group, the ALMPE showed increased IgG production 32-fold at 38 days, while the ALPE and AS04 only increased IgG production by 21-fold and 22-fold at 38 days. Then, ELISPOT assay was carried out to evaluate production of IFN-γ by splenocytes. No significant difference in IFN-γ secretion was observed between groups of ALMPE and Freund’s adjuvant (Figure 11c). Consequently, the results indicated the effective immune enhancement response of ALMPE formulation in M.RCAg-1 vaccinations.
Figure 11. M.RCAg-1 vaccination. Production of M.RCAg-1 antigen-specific antibodies in the serum at day 28 (a) and day 38 (b). ELISPOT assay on IFN-γ spot-forming cells among splenocytes (c,d). “Ag” represents the individual antigen group. Data are expressed as mean ± s.e.m. (n = 6). * p < 0.05, ** p < 0.01, *** p < 0.005.
2.5. Safety and Biocompatibility
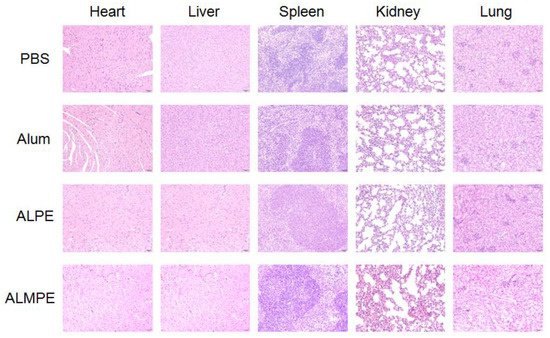
The safety and biocompatibility of ALPE and ALMPE was evaluated by analyzing the key factors such as serum biochemical parameters and histological changes of important organs, taking the PBS injection group as the blank control group. Blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alanine aminotransferase (ALP) of all groups were in the healthy level (
Table S1, Supplemental Data). Moreover, a histological analysis of major organs, such as heart, liver, spleen, kidney, and lung showed no evident difference among the all groups (
Figure 12). On the whole, compared with Freund’s adjuvant, which had obvious side effects and thus is not possible to use as a human vaccine [
32,
33], ALPE and ALMPE were verified with excellent biocompatibility.
Figure 12. Biocompatibility evaluations via histological analysis of major organs from C57BL/6 mice after 14 day of immunization.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines9111244