Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Bromelain, a mixture of proteases in pineapple rhizome, has beneficial biological properties. Following absorption, the compound remains biologically active in mammalian blood and tissues. Bromelain has multiple clinical and therapeutic applications because of its anti-arthritic activities.
- anti-arthritis
- anti-inflammation
- bromelain
1. Introduction
Among musculoskeletal disorders, osteoarthritis (OA) and rheumatoid arthritis (RA) are the most common prevalent disorders of the articular joints, and they cause disability and increase medical costs in elderly people. Proinflammatory cytokine-induced inflammation is the hallmark of both OA and RA.
OA is a whole-joint disease resulting in alteration of the structure of hyaline cartilage, subchondral bone, ligaments, capsules, synovial tissues, and periarticular muscles. Among proinflammatory cytokines, interleukin (IL)-1β participates in the pathogenesis of OA by upregulating matrix-degrading degraded enzymes (matrix metalloproteinases (MMPs), ADAMTS) and suppressing matrix molecules synthesis [1,2]. RA is typified by chronic inflammation of the lining of the joints with synovial inflammation and hyperplasia, hyaline cartilage degradation, bone destruction, and systemic features, including cardiovascular, pulmonary, psychological, and skeletal disorders. Tumor necrosis factor (TNF)-α is a proinflammatory cytokine plays a pivotal role in regulating the inflammatory response in RA [3].
Bromelain (EC 3.4.22.32), a proteolytic enzyme, was isolated from pineapple (Ananas comosus) rhizome. It has been recognized as a safe therapeutic phytochemical that exhibits clinical applications including fibrinolytic, antiedematous, antithrombotic, and anti-inflammatory effects [4,5]. The anti-inflammatory effects of bromelain were previously reported in pathological condition and cell types, such as the reduction of IL-1β, IL-6, and TNF-α secretion in immune cells [6] and downregulation of TNF-α receptor in a rat colitis model [7]. Meanwhile, IL-1β and TNF-α are the targets for OA and RA treatment, respectively. Bromelain was previously examined in a series of case reports of moderate OA and RA [8]. In OA, the pain and inflammation were reduced by oral bromelain, trypsin, and rutin administration in patients with OA [9].
2. Cytotoxicity of Bromelain Extract in Porcine Chondrocytes and Synovial Fibroblasts
Chondrocytes are the residence cells in cartilage, and they participate in OA pathogenesis. The cartilage explants model has been thoroughly used to study the pathology and therapeutic treatment of OA. Chondrocyte lysis mediated by bromelain extract was measured by lactate dehydrogenase (LDH) activity in culture medium. In this study, LDH activity was represented by the LDH levels, and the results illustrated that 35 days of exposure to bromelain extract (1–100 µg/mL) did not significantly increase LDH secretion from chondrocytes in porcine cartilage versus the untreated control. Meanwhile, LDH release was significantly higher following 30 mM H2O2 treatment (positive control) than in the untreated group (Figure 2A) Thus, bromelain extract (1–100 µg/mL) did not damage chondrocytes in cartilage, making it useful for generating a porcine cartilage explant model.
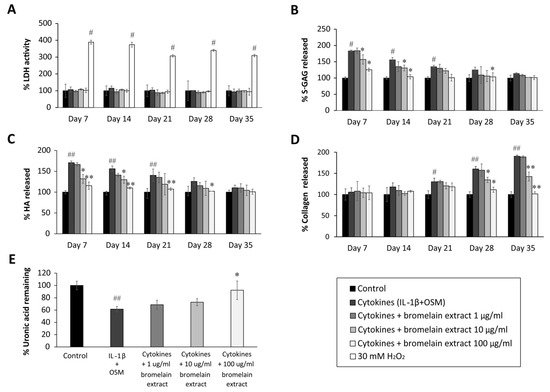
Figure 2. Effect of bromelain extract on the release of glycosaminoglycan release and matrix uronic acid content in porcine explants treated with cytokines (IL-1β/OSM) for 35 days. The culture medium was collected on days 0, 7, 14, 21, 28, and 35 from each group, and cartilage discs on day 35 were digested with 10 U of papain. The LDH activity in culture medium (A) was analyzed to determine the toxicity of bromelain compared with H2O2 treatment. The levels of s-GAG (B), HA (C), collagen released into culture medium (D), and uronic acid remaining in cartilage tissue (E) were measured and calculated as the percent change versus control. Each value is expressed as the mean ± SD. # p < 0.05 compared with control, * p < 0.05 compared with IL-1β treatment alone.
Synovial fibroblasts participate in RA pathogenesis. Synovial fibroblasts exposed to inflammatory cytokines trigger joint inflammation and cartilage and bone destruction in RA [13]. Human synovial sarcoma cells (SW982) were used to examine the effects of bromelain on TNF-α–mediated inflammation in this study. This cell line is an alternative in vitro model for studying the effects of anti-inflammatory drugs [14,15] and phytochemicals [16] on RA. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was assessed to determine the cytotoxicity of bromelain extract in SW982 cells, and the results illustrated that treatment with bromelain extract (1.25–200 µg/mL) for 24–72 h caused no significant cytotoxic effects. The percent cell viability under all conditions relative to the control exceeded 80% (Figure 3A). Thus, this range of bromelain concentrations was used for cell culture experiments.
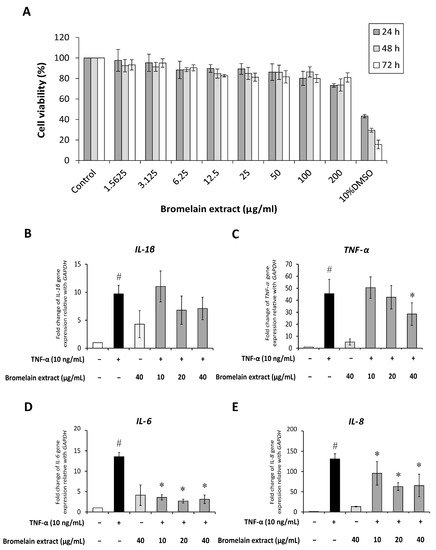
Figure 3. Effect of bromelain extract on cytotoxicity and inflammatory gene expression. The cytotoxicity of bromelain extract in SW982 cells was assessed by the MTT assay (A). The effects of bromelain on the gene expression of inflammatory cytokines were investigated by cotreatment with 10 ng/mL human recombinant TNF-α and bromelain extract (10–40 µg/mL) for 4 h. The gene expression of IL-1β (B), TNF-α (C), IL-6 (D), and IL-8 (E) was measured by quantitative RT-PCR. Each value is expressed as the mean ± SD. # p < 0.05 compared with control, * p < 0.05 compared with TNF-α treatment alone.
3. Chondroprotective Effects of Bromelain Extract
IL-1β induces inflammation in OA, but an in vitro study previously demonstrated that IL-1β, alone, did not induce collagen degradation [17]. In arthritis, oncostatin M (OSM), a cytokine present in synovial fluid and joint tissue, synergizes the action of other inflammatory cytokines (e.g., IL-1, TNF-α, IL-17, lipopolysaccharide (LPS)) [18]. This cytokine participates in inflammatory processes in bone, cartilage, lung, vesicular, and skin diseases [19]. To induce inflammation in this study, porcine cartilage discs were treated with IL-1β/OSM and cotreated with bromelain extract for 35 days. Cartilage explant models are widely used to study the degenerative changes in cartilage tissue [20,21] and therapeutic mechanism [22,23]. In this model, the release of glycosaminoglycans (GAGs) from explants into the cultured medium and the low content of uronic acid and collagen in cartilage explants reflect the induced cartilage degradation.
The sulfated glycosaminoglycan (s-GAG) level in the conditioned medium of IL-1β/OSM-treated cartilage explants was significantly elevated in the early inflammatory stage between days 7 and 14 (Figure 2B) compared with the control level. After cotreatment with bromelain extract (1–100 µg/mL) in the presence of IL-1β/OSM, the results illustrated that high concentrations of bromelain extract (10 and 100 µg/mL) significantly decreased s-GAG release in the cultured medium on days 7–21 compared with the findings in the IL-1β/OSM treatment group. IL-1β/OSM significantly increased the release of hyaluronic acid (HA), the major non–s-GAG, in cultured medium on days 7, 14, and 21 (1.7-, 1.5-, and 1.4-fold, respectively) compared with the control level, and high concentrations of bromelain extract (10–100 µg/mL) significantly inhibited the release of HA on days 7–28 compared with the results in the IL-1β/OSM treatment group (Figure 2C).
The release of degraded collagen in culture medium was significantly higher in the IL-1β/OSM treatment group than in the control group on days 21–35 (Figure 2D). Bromelain cotreatment (10–100 µg/mL) significantly decreased the release of degraded collagen on days 28 and 35 compared with the findings in the IL-1β/OSM treatment group. On day 35, papain-digested cartilage discs exhibited significantly decreased levels of uronic acid (approximately 40%), which influences matrix GAG degradation, in the IL-1β/OSM treatment versus the control level. Upon cotreatment with bromelain extract (100 µg/mL), the uronic acid level in porcine cartilage tissue was significantly higher than that in the IL-1β/OSM treatment group (Figure 2E).
GAGs (s-GAG and HA) and collagen in cartilage matrix are degraded in response to IL-1β/OSM-induced MMP production and activation [24,25,26]. The reduction of IL-1β/OSM-mediated extracellular matrix degradation in the culture medium indicated the chondroprotective property of bromelain extract. In addition, this chondroprotective effect was confirmed by the higher cartilage uronic acid content in the presence of bromelain extract. In clinical trials, oral bromelain was previously described as an analgesic and anti-inflammatory agent for OA and RA [27,28]. Regarding the inflammatory process, the effects of bromelain were reported to involve the reduction of prostaglandin E2 levels [29]. This is the first report to demonstrate the mechanism by which bromelain inhibits cartilage degradation in an ex vivo OA model.
4. Discussion
Oral administration of bromelain (12 g/day) can be absorbed with no side effects, and about 40% of the high molecular weight molecules are found in blood circulation [47] Bromelain is absorbed via the human intestines in its intact form with a half-life of approximately 6–9 h [5]. The blood concentration of bromelain peaked 1 h after administration in prior research [48]. The proteolytic activity of bromelain was detected in plasma and was found in the bound form with antiproteinases (2-macroglobulin and alpha1-antichymotrypsin) [49].
In previous research, bromelain extract from pineapple rhizome was characterized and revealed to exhibit protease activity with a molecular weight of approximately 25–27 kDa [10]. In this study, the HPLC profile illustrated that the bromelain extract was identical to commercially available stem bromelain with protease activity and many beneficial biological and pharmacological activities [50].
Regarding joint disease, oral bromelain administration significantly was demonstrated to decrease pain and stiffness in patients with knee OA [51]. The pharmacological mechanism of bromelain was reported as the analgesic influence on bradykinin [52,53], which is a pain-producing substance. In addition, the reduction of pain was an indirect effect of anti-inflammatory actions such as the reduction of edema, debris, and immune responses. A clinical study demonstrated that bromelain produced a significant to complete decrease in soft tissue swelling in patients with arthritic joint swelling [54].
A number of clinical trials also demonstrated that oral administration of bromelain is safe, and it has been used as a food supplement and alternative to NSAIDs in patients with acute inflammation and sports injuries [27]. The antiapoptotic and anti-inflammatory effects of bromelain were demonstrated in primary canine [55] and chondrocyte cell line, SW1353 [56]. However, the molecular mechanism of bromelain in joint resident cells has not yet been reported.
The results of our study supported the anti-inflammatory effects of bromelain on OA and RA. In our OA model, bromelain extract exerted chondroprotective effects against IL-1β by suppressing GAG and collagen degradation. Cartilage extracellular matrix degradation is mediated by MMP overexpression and activation. The degraded molecules are released from cartilage to synovial fluid in addition to the loss of the biological function of cartilage. The suppressive effects of bromelain extract on IL-1β activity and porcine cartilage degradation demonstrated its potential benefits for OA treatment. In an RA model, bromelain extract attenuated TNF-α–induced inflammatory cytokine expression by inhibiting NF-κB and MAPK signaling. The inflammation in synovial fibroblasts is mediated by the TNF-α signaling pathway. The release of proinflammatory cytokines from synovial fibroblasts potentiates the RA pathological process. The blockade of TNF signaling is a clinically verified strategy for treating RA. The mechanism by which bromelain suppresses inflammation in SW982 cells could explain the therapeutic effects of bromelain against RA.
The best biological materials for studying arthritic pathology are human cartilage and primary synovial fibroblasts. Histological analysis of cartilage extracellular matrix molecules and MMPs in IL-1β–treated porcine cartilage tissue should be performed for confirm the chondroprotective effect of bromelain. The protein expression of cytokines released from SW982 cells should be analyzed to verify the anti-inflammatory effects of bromelain. To further investigate the antiarthritic activity of bromelain, an in vivo study using an animal model and investigation of the expression of MMPs and cytokines should be conducted. The findings in this study further support the safety, analgesic activity, and anti-inflammatory effects of bromelain in the treatment of OA and RA.
This entry is adapted from the peer-reviewed paper 10.3390/plants10112273
This entry is offline, you can click here to edit this entry!
