Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Food waste (FW) is already acknowledged as a major global issue that threatens the long-term viability of the food supply chain.
- fruit waste
- vegetable waste
- waste valorization
- bioactive compound
1. Introduction
Food waste (FW) is already acknowledged as a major global issue that threatens the long-term viability of the food supply chain [1]. FW in the European Union is estimated to be at 89 million tonnes per year. This is expected to rise by 40% in the next four years [2]. According to the Food and Agriculture Organization of the United Nations [3] and the International Food Policy Research Institute [4] estimated one-third of food produced globally, or 1.3 billion tonnes, is thrown away each year. Over half is generated at the final consumption stage in food services (e.g., restaurants, school canteens) and households [5][6]. The Sustainable Development Goals (SDGs) recognize the importance of this issue. By 2030, SDG Target 12.3 calls for the reduction by half of the per-capita global FW at retail and consumer levels, diminishing food losses in production and supply chains, including post-harvest losses [7]. The call was agreed to by all 193 UN member states, which comes as no surprise.
Every year, more than 1748 million tonnes of fruit and vegetable waste (FVW) are produced worldwide [8]. Due to rapid economic growth, FW generation in Asia is predicted to increase by 278 to 416 million tonnes [9], resulting in an increase in world anthropogenic (carbon) emissions [10]. Malaysia is expected to produce 6.7 million tonnes of FW per year by 2020 [11]. In the EU, households account for over half of all FW [12]. Around 12 percent of the total EU food production is wasted in the United Kingdom [13], while the United States recorded the highest FW rates per capita at 278 kg [14]. FVW is a major component of FW, particularly in industrialized countries [15][16]. Figure 1 shows the FW management practices and the novel or emerging valorization approaches [17].
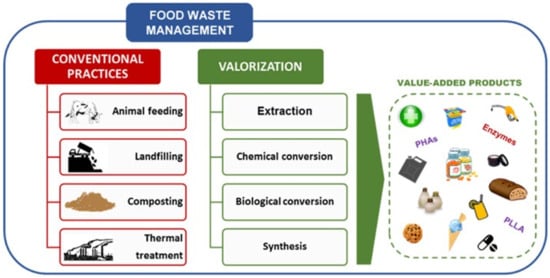
Figure 1. Food waste management practices and the novel or emerging valorization methods. Reproduced with permission from Ref. [17]. Copyright 2021 Elsevier.
According to Fabi et al. [18], median losses are estimated to be higher than 10 percent in Africa and Latin America for fruits and vegetables. In comparison, they range between 4 and 7 percent in Europe and North America. Figure 2 depicts the amounts of fruits produced globally, including 124.73 million metric tonnes (MMT) of citrus, 114.08 MMT of bananas, 84.63 MMT of apples, 74.49 MMT of grapes, 45.22 MMT of mangoes, mangosteens, and guavas, and 25.43 MMT of pineapples. Besides the production of potatoes, which was recorded at a considerable 3820.00 MMT, other vegetables are also shown in Figure 2, including tomatoes (171.00 MMT), cabbages and other brassicas (71.77 MMT), carrots and turnips (38.83 MMT), cauliflowers, broccolis (24.17 MMT), and peas (17.42 MMT) [19]. Due to losses in sales and transportation costs, such wastage, including subsequent generation of FVW, raises market operation costs, resulting in higher inflation.
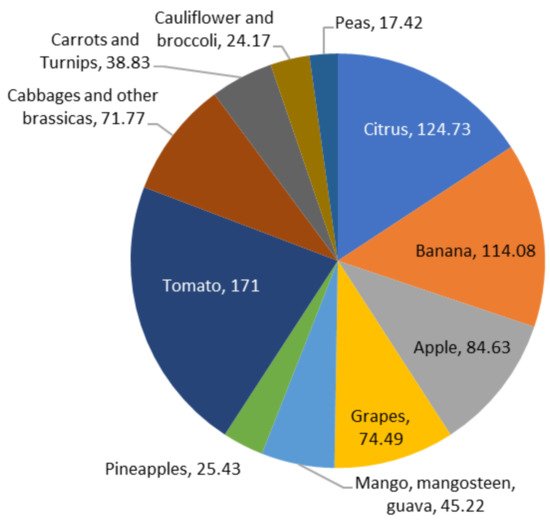
Figure 2. A significant amount of fruits and vegetables (in MMT) are produced globally.
The dairy, meat, fishery, and seafood processing sectors are the primary contributors of trash from animal wastes. Numerous types of residues can be identified from vegetable wastes, including grains, roots and tubers, oil crops and pulses, and fruits and vegetables, depending on the source [20]. Typically, FVW is characterized by a high water content and a high concentration of biodegradable organic substances (e.g., carbohydrates, lipids, and organic acids) [21]. As a result of these varied organic wastes, typical solid waste management systems (such as landfills and incineration) may cause serious environmental impacts, such as the discharge of leachate and the production of greenhouse gases [22].
FVW is generated in large quantities in open markets, but little information is available on the actual volume of waste generated. Since significant amounts of waste are produced by the fruit and vegetable value chains, biorefinery concepts have been developed to valorize these wastes [23]. Waste from the FVW can be reused or recycled, which is more environmentally beneficial than disposal through open dumpsites or incineration [24]. Wholesale marketplaces, supermarkets, and agricultural activities are the traditional sources of FVW. Fruits and vegetables produce FVW at every stage of the chain, including production, transportation, storage, distribution, and consumption [25]. The valorization of FVW and potential products are shown in Figure 3.
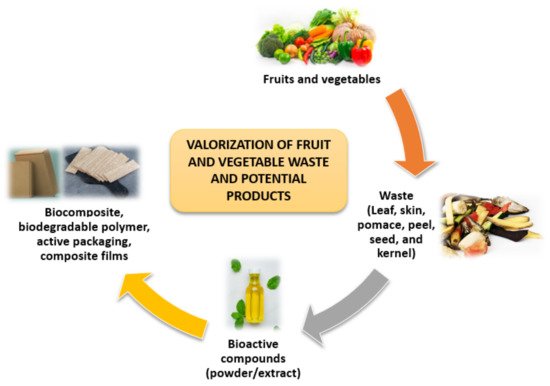
Figure 3. Valorization of FVW and potential products.
There are several reviews on the applications of fruit and vegetable wastes. A review conducted by Bayram et al. [26] focused on the potential applications of fruit- and vegetable-based by-products such as biopolymers, biocomposites, active or intelligent packaging, and edible films and coatings. The authors discussed the advantages, disadvantages, and applications of these by-products as well. Kadzińska et al. [27] reviewed the role of specific chemical compounds found in fruits and vegetables, particularly in designing the physicochemical and functional properties of edible packaging materials. The advantages and disadvantages of using fruit and vegetables as components in matrix-forming solutions and their potential applications, future trends, and issues to consider when commercializing these products were discussed within the context of sustainable development.
In the fruit and vegetable processing industry, by-products such as peels, seeds, and shells are produced in large quantities [28][29]. These by-products contain high concentration of bioactive components such as antioxidants (polyphenols and dietary fibers), pigments and flavor compounds, proteins, essential oils, enzymes, and dietary fibers [30]. Coman et al. [31] looked at the bioactive potential of fruit and vegetable by-products and how they could be used in the food industry (functional foods) or the health sector (nutraceuticals). Several applications of food vegetable waste incorporated into the meat and its derivatives were reviewed by Calderón-Oliver and López-Hernández [32]. The peels and seeds of fruits, such as grapes, pomegranates, avocados, and citrus, are the most commonly used vegetable by-products because they help inhibit oxidation (lipid and protein) and the growth of pathogenic and deteriorating bacteria in the food supply. Adding these by-products to meat products can sometimes improve the quality of the product while also extending its shelf life. In food processing of waste and biomass by-products, pectin is one of the most common constituents; therefore, improving pectin extraction and recovery is critical to completely valorize these significant feedstock resources [29]. As a result, it is crucial to investigate the composting process of FW, particularly FVW, within this framework.
Given the potential for the valorization of such organic waste, research on the proper use of waste materials obtained from horticulture commodities may generate sustainable development initiatives to minimize environmental problems [19]. Significant products can be produced from these organic wastes, including active packaging, biopolymers, biocomposites, and other by-products.
2. Active Packaging and pH Indicator Film
2.1. Active Packaging
The integration of additives into the packaging system to maintain or enhance the quality of food products and shelf life is referred to as active packaging [33]. Active packaging concepts include moisture absorbers, gas scavengers, carbon dioxide emitters, antioxidant, and antimicrobial-releasing and-containing systems. For example, the addition of antimicrobial additives into the packaging materials can minimize the risk of food spoilage and contamination from microorganisms [34]. Several additives have been successfully incorporated into packaging materials, including organic acids and their salts, bacteriocins, enzymes, chelators, and a range of plant extracts [35][36][37]. Due to concern about the health hazards of synthetic additives, researchers have been implementing various natural plant extracts to the biopolymers as active components [38]. In addition, active ingredients from FVW have added another level to active packaging systems as a solution for reducing FW. Several studies have focused on utilizing these wastes to make active films and investigated their properties, as summarized in Table 1.
Table 1. Utilization of fruit and vegetable wastes in food packaging systems.
| Film Type | Polymers | Fruit/Vegetable Waste | Applications | Findings | Ref. |
|---|---|---|---|---|---|
| Active film | Hydroxypropyl high-amylose starch | Pomegranate peel (PGP) | - | PGP inhibited the growth of S. aureus and Salmonella bacteria. | [34] |
| Active film | Cassava starch | Blueberry pomace (BP) | - | BP improved the barrier properties and released active compounds into acidic medium. | [39] |
| Active film | Gelatin capsule waste | Fiber and ethanolic extract from blueberry juice processing waste | Sunflower oil | Improved light barrier properties; reduced the lipid oxidation of sunflower oil and stability in antioxidant activity. | [40] |
| Active film | Tapioca starch thymol |
Jackfruit skin and straw as filler | Cherry tomato | Jackfruit skin and straw resulted in higher tensile strength with lower water solubility and water vapor permeability. | [41] |
| Active packaging | Poly (butylene adipate-co-terephthalate) (PBAT) Cinnamon essential oil |
Cellulose nanofibers (CNF) | Strawberry | High thermal stability, decreased water vapor permeability. | [37] |
| Active packaging | Chitosan | Apricot kernel essential oil (AKEO) | Bread slices | AKEO improved water resistance, water vapor barrier, and mechanical properties. Inhibited E. coli, B. subtilis and fungal growth. | [42] |
| Biodegradable film | Tapioca starch | Banana pseudostems (BP) | - | BP reduced the mechanical and optical properties, improved the barrier properties. | [43] |
| Active film | Mango kernel fat (MKF), phenolic extract from mango kernel (MKPE), mango kernel starch (MKS), |
Mango kernel | - | Films exhibited antioxidant activity, UV-absorbing capacity, and good barrier properties. |
[44] |
| Biodegradable film | Pectin, sodium carboxymethyl cellulose (CMC− Na) Thyme essential oil |
Okara soluble dietary fiber | - | Higher mechanical, barrier, optical, and antioxidant properties. Antimicrobial activity against E. coli and S. aureus was not significant. | [45] |
| Active edible film | Basil seed gum Zataria multiflora essential oil |
Basil seed gum (BSG) | - | Efficient antimicrobial activity against E. coli and B. cereus. | [46] |
For example, Luchese et al. [39] added blueberry pomace into cassava starch films. The aromatic compounds in blueberry pomace improved the light barrier properties, indicating the films’ ability to preserve food against UV lights. At the same time, the films were structurally stable when immersed in water for more than 24 h. The authors suggested the feasibility of the films for packaging aqueous food products.
In another study, fiber and ethanolic extracts from blueberry juice from processing waste were used to make active films from gelatin capsules wastes [40]. The study found that the films with fiber showed reduced tensile strength and increased water vapor permeability with improved light barrier activity against UV light, which effectively reduced the lipid oxidation of sunflower oil. Both films showed significant decreases in light transmission and stability in antioxidant activity over 28 days.
Ali et al. [34] utilized pomegranate peel as a filler and an antimicrobial agent in developing films with hydroxypropyl high-amylose starch. The results showed that the tensile properties of the films improved and the pomegranate peel inhibited the growth of both Gram-positive (S. aureus) and Gram-negative (Salmonella) bacteria. On the other hand, research done by dos Santos Caetano et al. [47] produced biodegradable films based on minimally processed pumpkin residue extract (PRE) (0 to 6%) incorporated with cassava starch, glycerol, and oregano essential oil (OEO) (0 to 2%). The addition of pumpkin residue is crucial, since it provides opacity to the films, but it was not effective in improving the antioxidant and antimicrobial properties.
Other researchers also used pomegranate peel as an antibacterial additive with sodium caseinate powder [48]. The peel resulted in a decrease in tensile strength and increased water vapor permeability (WVP). The films showed profound growth inhibition for Gram-positive (S. aureus) rather than Gram-negative (E. coli) bacteria. Shukor et al. [41] prepared tapioca-starch-based films incorporating thymol, jackfruit skin, and straw. Improvement in mechanical and barrier properties was observed for the films. The incorporation of skin and thymol retarded bacterial and fungal growth due to the antimicrobial activity of the films.
2.2. pH Indicator Films
Researchers are currently attracted to intelligent packaging systems, whereby active ingredients such as dyes and pigments are added into the film as pH indicators to trace and monitor food freshness throughout the storage period. This is related to the interactions between the food and its environment and the packaging material [49]. Table 2 shows some of the fruit and vegetables used as pH indicators in packaging films.
Table 2. Utilization of fruit and vegetable wastes as pH indicator in packaging films.
| Polymers | Fruit/Vegetable Waste | Applications | Findings | Ref. |
|---|---|---|---|---|
| Cassava starch | Blueberry residue (BR) powder, two particle sizes | Distilled water, sucrose, sodium chloride, soybean protein, milk protein, whole milk powder, orange juice, corn oil, chicken meat | BR films displayed changes in color responding to the pH of tested samples. | [50] |
| Corn starch | Blueberry juice processing waste | - | The blueberry residue film showed visual color differences in different pH ranges. | [51] |
| Sweet potato starch | Sweet potato peel | Chicken flesh | SPP reduced the mechanical and barrier properties, did not influence the color response of the films. | [52] |
The colorimetric pH indicator films display apparent color changes with alterations of the food pH due to food deterioration and extrinsic environmental changes [53]; thus, from these color changes, the consumers receive authentic information regarding the food’s quality and its edibility, which is not achievable from the expiry date written on the package. As a pH indicator dye replacing synthetic pigments, natural dyes are now used mainly in biodegradable packaging materials [54][55]. Interestingly, the natural dyes and pigments from waste and by-products from fruits and vegetables have also been implemented as pH sensing dyes into packaging films to ensure food safety [56].
A study was conducted by developing colorimetric indicator film from mulberry based on gelatin and polyvinyl alcohol (PVA), whereby anthocyanin extract from the residue of mulberry processing was incorporated. This study showed that the mechanical properties were improved and visible color changes were shown when monitoring fish spoilage [57]. In addition, an earlier study produced films from cassava starch and blueberry residue that were rich in anthocyanin. Insertion of blueberry residue produced less compact films with high oxygen permeability. The films exhibited visual color changes in the pH range of 2 to 12 [56]. Luchese et al. [50] developed biodegradable pH indicator films based on cassava starch, utilizing the blueberry residue obtained after juice processing. The researchers deliberated on two different particle sizes for the blueberry residue powder for film preparation. They found that the films with smaller particles were more uniform and homogenous in appearance and the color change with pH was more intense than films with large particles.
In addition, the tensile strength of the films decreased whereas elongation increased; simultaneously, the water vapor permeability of the films increased due to the presence of the particles and their heterogeneity. In another study, blueberry agro-industrial waste, a co-product from juice processing, was successfully used to develop pH indicator films based on corn starch [51]. The films changed their colors in response to different pH, and the color changes were visually perceptible to the human eye.
Black chokeberry (Aronia melanocarpa) pomace extract (a residue material after juice pressing) was chosen as a pH sensing dye of chitosan films. The addition of pomace extract reduced the solubility and swelling of chitosan, and these indicator films maintained integrity in acidic pH environments [58]. Sohany et al. [52] utilized sweet potato peel powder (SPP) as a filler to develop sweet potato starch (SPS)-based pH indicator films incorporating purple sweet potato anthocyanin. The films with peel exhibited reduced tensile strength with higher swelling and WVP values. Visually, the films were dark maroon and changed their color in response to various pH buffers, as shown in Figure 4. The films successfully indicated chicken freshness by changing their color with changes in pH of the deteriorated chicken.

Figure 4. Visual appearance of SPS and SPS/SPP films at CA of 0, 1, 1.5, and 2%. Reproduced with permission from Ref. [52]. Copyright 2021 Taylor & Francis.
This entry is adapted from the peer-reviewed paper 10.3390/polym13203503
References
- Tonini, D.; Albizzati, P.F.; Astrup, T.F. Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manag. 2018, 76, 744–766.
- Stenmarck, Â.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016.
- FAO. The State of Food and Agriculture 2019: Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; pp. 2–13.
- International Food Policy Research Institute. 2019 Global Food Policy Report; International Food Policy Research Institute: Washington, DC, USA, 2019.
- Fiore, M.; Pellegrini, G.; Sala, P.L.; Conte, A.; Liu, B. Attitude toward food waste reduction: The case of Italian consumers. Int. J. Glob. Small Bus. 2017, 9, 185–201.
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security–Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20.
- FAO. Tracking Progress on Food and Agriculture-Related SDG Indicators 2020—A Report on the Indicators under FAO Custodianship; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020.
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Triolo, J.M.; Flotats, X.; de Mendonça Costa, M.S.S. Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag. 2018, 71, 618–625.
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138.
- Yang, J.; Cai, W.; Ma, M.; Li, L.; Liu, C.; Ma, X.; Li, L.; Chen, X. Driving forces of China’s CO2 emissions from energy consumption based on Kaya-LMDI methods. Sci. Total. Environ. 2020, 711, 134569.
- Bong, C.P.C.; Ho, W.S.; Hashim, H.; Lim, J.S.; Ho, C.S.; Tan, W.S.P.; Lee, C.T. Review on the renewable energy and solid waste management policies towards biogas development in Malaysia. Renew. Sustain. Energy Rev. 2017, 70, 988–998.
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251.
- Barrera, E.L.; Hertel, T. Global food waste across the income spectrum: Implications for food prices, production and resource use. Food Policy 2021, 98, 101874.
- Brizi, A.; Biraglia, A. “Do I have enough food”? How need for cognitive closure and gender impact stockpiling and food waste during the COVID-19 pandemic: A cross-national study in India and the United States of America. Personal. Individ. Differ. 2021, 168, 110396.
- Li, Y.; Wang, L.-E.; Liu, G.; Cheng, S. Rural household food waste characteristics and driving factors in China. Resour. Conserv. Recycl. 2021, 164, 105209.
- McCarthy, B.; Kapetanaki, A.B.; Wang, P. Completing the food waste management loop: Is there market potential for value-added surplus products (VASP)? J. Clean. Prod. 2020, 256, 120435.
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510.
- Fabi, C.; Cachia, F.; Conforti, P.; English, A.; Moncayo, J.R. Improving data on food losses and waste: From theory to practice. Food Policy 2021, 98, 101934.
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531.
- Álvarez, C.; Mullen, A.M.; Pojić, M.; Hadnađev, T.D.; Papageorgiou, M. Classification and target compounds. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–49.
- Li, W.; Bhat, S.A.; Li, J.; Cui, G.; Wei, Y.; Yamada, T.; Li, F. Effect of excess activated sludge on vermicomposting of fruit and vegetable waste by using novel vermireactor. Bioresour. Technol. 2020, 302, 122816.
- Chen, D.M.-C.; Bodirsky, B.L.; Krueger, T.; Mishra, A.; Popp, A. The world’s growing municipal solid waste: Trends and impacts. Environ. Res. Lett. 2020, 15, 074021.
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.-M.; Kopsahelis, N.; de Castro, A.M.; Freire, D.M.G.; Nychas, G.-J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2, 3-butanediol. Food Bioprod. Process. 2018, 108, 27–36.
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59.
- Chatterjee, B.; Mazumder, D. New approach of characterizing fruit and vegetable waste (FVW) to ascertain its biological stabilization via two-stage anaerobic digestion (AD). Biomass Bioenergy 2020, 139, 105594.
- Bayram, B.; Ozkan, G.; Kostka, T.; Capanoglu, E.; Esatbeyoglu, T. Valorization and Application of Fruit and Vegetable Wastes and By-Products for Food Packaging Materials. Molecules 2021, 26, 4031.
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An overview of fruit and vegetable edible packaging materials. Packag. Technol. Sci. 2019, 32, 483–495.
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515.
- Figueroa, K.H.N.; García, N.V.M.; Vega, R.C. Cocoa By-Products. In Food Wastes and By-Products: Nutraceutical and Health Potential; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 373–411.
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416.
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 91, pp. 157–225.
- Calderón-Oliver, M.; López-Hernández, L.H. Food vegetable and fruit waste used in meat products. Food Rev. Int. 2020, 1–27.
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810.
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126.
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458.
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Walkiewicz, A.; Pertile, G.; Frąc, M.; Cieślak, K.J.; Zdunek, A. Evaluation of nanocomposite made of polylactic acid and nanocellulose from carrot pomace modified with silver nanoparticles. Polymers 2020, 12, 812.
- Montero, Y.; Souza, A.G.; Oliveira, É.R.; dos Santos Rosa, D. Nanocellulose functionalized with cinnamon essential oil: A potential application in active biodegradable packaging for strawberry. Sustain. Mater. Technol. 2021, 29, e00289.
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120.
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839.
- De Moraes Crizel, T.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crop. Prod. 2016, 87, 218–228.
- Shukor, U.; Nordin, N.; Tawakkal, I.; Talib, R.; Othman, S. Utilization of jackfruit peel waste for the production of biodegradable and active antimicrobial packaging films. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–192.
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166.
- Othman, S.H.; Tarmiti, N.A.N.; Shapi’i, R.A.; Zahiruddin, S.M.M.; Tawakkal, I.S.M.A.; Basha, R.K. Starch/banana pseudostem biocomposite films for potential food packaging applications. BioResources 2020, 15, 3984–3998.
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619.
- Lin, D.; Zheng, Y.; Wang, X.; Huang, Y.; Ni, L.; Chen, X.; Wu, Z.; Huang, C.; Yi, Q.; Li, J. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int. J. Biol. Macromol. 2020, 165, 1241–1249.
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103.
- Dos Santos Caetano, K.; Lopes, N.A.; Costa, T.M.H.; Brandelli, A.; Rodrigues, E.; Flôres, S.H.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147.
- Emam-Djomeh, Z.; Moghaddam, A.; Yasini Ardakani, S.A. Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packag. Technol. Sci. 2015, 28, 869–881.
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century–A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022.
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218.
- Luchese, C.L.; Sperotto, N.; Spada, J.C.; Tessaro, I.C. Effect of blueberry agro-industrial waste addition to corn starch-based films for the production of a pH-indicator film. Int. J. Biol. Macromol. 2017, 104, 11–18.
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005.
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280.
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438.
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent pH-sensitive indicator based on starch-cellulose and alizarin dye to track freshness of rainbow trout fillet. Int. J. Biol. Macromol. 2019, 132, 157–165.
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324.
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604.
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193.
This entry is offline, you can click here to edit this entry!
