Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Area Studies
Erectile dysfunction (ED) is an increasing disorder [16], affects 25 to 35 million men over 18 years in Europe. Pharmaceuticals used to reduce this disorder act as phosphodiesterase-5 (PDE-5) inhibitors, a family of enzymes typically active in cyclic guanosine monophosphate (cGMP) degradation. The inhibition of PDE-5 results in the intracellular accumulation of cGMP, which plays a central role in signal transduction and regulates several physiological responses.
- phosphodiesterase-5 (PDE-5) inhibitors
- environment contamination
- analytical methods
1. Introduction
Emerging pollutants (EPs) are synthetic or naturally occurring compounds not commonly examined in the environment [1]. Still, they can enter ecosystems and cause recognised or supposed adverse effects on ecology and human health [1]. Moreover, they undergo chemical/biological transformations, forming by-products sometimes more toxic than the parent molecules. The final issue is the accumulation of original and transformed substances in water bodies [2]. In particular, Zuccato et al. [3] evidenced the presence in ground and surface waters of drugs and pharmaceuticals having polar structures, probably coming from WWTP effluents.
When absorbed by the body, a pharmaceutical substance enters the circulation and distributes to reach the target site to perform its function [2][4]. When the metabolism process is activated, some molecules reach the target site and others transform into inactive metabolites, which are substances no longer producing effects into the body. Many medicines, however, are excreted without being metabolised or at least without being completely inactivated [2][5]. These, together with sewage, reach the WWTP, where organic loads degrade and water is purified. Unfortunately, these structures are often not designed to degrade active substances of pharmaceutical origin, which, therefore, once again manage to resist, unharmed, and maintain their effectiveness. As a result, the purified water (still rich in active ingredients) flows into the receiving channels, carrying a load of pollutants to rivers and lakes. Thus, tons of active substances such as antibiotics, anti-neoplastic, estrogens and others are poured into surface waters [6][7]. Once in the environment, the drug eventually degrades or can persist very long, resulting in noticeable build-ups [8]. In the sediments of some Italian rivers such as the Po, Lambro and Adda rivers, as well as in the aqueducts of the towns of Varese and Lodi, traces of various drugs were present in different amounts, including antibiotics (lincomycin and erythromycin), anticancer (cyclophosphamide), anti-inflammatory (ibuprofen), diuretics (furosemide) and antihypertensive (atenolol) drugs [9]. The existence of these compounds in environmental systems is of concern since they constitute a complex assortment, which could induce the occurrence of undesirable synergistic effects and could be responsible for many health adverse effects such as allergies, development of antibiotic-resistance phenomena, disorders of the endocrine system, cytolytic or cytostatic effects and others [10][11][12].
2. Source of PDE-5 Inhibitors in the Environment
According to the “anatomical therapeutic and chemical” (ATC) classification system, PDE-5 inhibitors are available in the class ATC code G04BE. The four most significant inhibitors used are sildenafil (Viagra®), tadalafil (Cialis®), vardenafil (Levitra®) and avanafil (Spedra®), all approved by Food and Drug Administration (FDA). In addition, there are other pharmaceuticals that are non-FDA approved and commercially available in some countries, such as udenafil (Zydena®) in South Korea and Malaysia, mirodenafil (Mvix®) in South Korea and lodenafil carbonate (Helleva®) in Brazil [13].
Sildenafil (C22H30N6O4S) was discovered in 1989 by the Pfizer Cardiovascular Research and Development Group (Sandwich, Kent, UK) during research focused on identifying PDE-5 inhibitors to treat angina pectoris due to the abundant presence of the PDE-5 enzymes in platelets and vascular smooth muscle cells. It showed low effectiveness on angina pectoris tests and penile erection as the primary collateral effect [14]. The FDA, in 1998, approved sildenafil for erectile dysfunction treatments and then in 2005, the European Medicine Agency (EMA) approved it for class II and class III pulmonary hypertension treatments. Vardenafil (C23H32N6O4S) and tadalafil (C22H19N3O4) were introduced clinically in 2003, while avanafil (C23H26ClN7O3) in 2013. Although the structural differences between these compounds are minor, they have different pharmacokinetic properties (absorption, distribution, metabolism and excretion). In addition, they can exert their activity also on other PDE-types, as reported in Table 1 [15].
Table 1. Pharmacokinetic parameters of PDE-5 inhibitors (re-edited from [15]).
| Parameters/Drugs | Sildenafil (Viagra) | Vardenafil (Levitra) | Tadalafil (Cialis) | Avanafil (Spedra) |
|---|---|---|---|---|
| Bioavailability | 41% (mean) 25–63% (range) |
15% (mean) | - | - |
| Tmax | 1 h (median) 0.5–2 h (range) |
1 h (median) 0.5–2 h (range) |
2 h (median) 0.5–6 h (range) |
0.5–0.75 h (range) |
| Protein binding | 96% | 95% | 94% | 99% |
| Metabolism | Major: CYP3A4 Minor: CYP2C9 |
Major: CYP3A4 Minor: CYP3A5, CYP2C |
CYP3A4 | Major: CYP3A4 Minor: CYP2C |
| Active metabolite(% effect) | Yes (20%) N-demethylation |
Yes (7%) Demethylation |
No | Yes (4%) Methylation, glucuronidation |
| Half-life(T1/2) | 4 h | 4–5 h | 17.5 h | 5 h |
| Elimination | 80% faeces 13% urine |
91–95% faeces 2–6% urine |
61% faeces 36% urine |
62% faeces 21% urine |
| Ingestion with high-fat meals | ↓ Cmax 29% ↑ Tmax by 1 h |
↓ Cmax 18–50% | Not affected | ↓ Cmax 24–39% ↑ Tmax by 1.12–1.25 h |
| Additional PDE inhibition | PDE1, PDE6 | PDE1, PDE6 | PDE11 | - |
Cmax = peak concentration; CYP = cytochrome P450; Tmax = time to peak concentration.
All four PDE-5 inhibitors are rapidly absorbed from the gastrointestinal tract and show broadly similar Tmax, except for tadalafil which has the longest Tmax. Peak plasma concentration (Cmax) of sildenafil is reached in less than 1 h [16]. Vardenafil and avanafil have similar pharmacokinetics to sildenafil, with Tmax approximately 1 h [17] and 30–45 min [18]. Tadalafil reaches its maximum concentration in plasma after about 2 h [19].
Sildenafil, vardenafil and avanafil have a terminal half-life (T1/2) of between 4 and 5 h, and tadalafil has a half-life of 17.5 h.
Huang et al. [15] reported that each PDE-5 inhibitor undergoes metabolism predominantly through the hepatic isoenzyme cytochrome P450 (CYP) 3A4 pathway. Minor pathways include CYP2C9 for sildenafil, CYP3A5 and CYP2C for vardenafil and CYP2C for avanafil. Of these four pharmaceuticals, only tadalafil produces human metabolites that are not pharmacologically active. Sildenafil predominantly metabolises into an N-desmethyl metabolite that contributes to approximately 20% of the parent molecule total pharmacological activity [20]. Vardenafil and avanafil produce active metabolites that contribute 7% [21] and 4% [22] of the real pharmacological action. All the PDE-5 inhibitors mainly excrete as metabolic by-products in the faeces and to a reduced amount in the urine.
Thanks to their different features, some PDE-5 inhibitors can be used in many clinical treatments and not only for erectile dysfunction treatments. For example, the national medicines agencies approved tadalafil for: (i) the treatment of lower urinary tract symptoms (LUTS); (ii) secondary to benign prostatic hyperplasia (BPH); and (iii) for the treatment of pulmonary arterial hypertension (PAH), a condition of increased blood pressure within the arteries of the lungs [19].
Sildenafil is approved for PAH treatment because it inhibits the PDE-5 in the pulmonary blood vessels and promotes the vasodilator action of nitric oxide by maintaining high cGMP levels [20].
In addition, researchers performing mice models with Alzheimer’s disease proved that PDE-5 inhibitors can effectively promote the cGMP-mediated processes involved in consolidating information in memory and countering the neurodegenerative mechanisms typical of Alzheimer’s disease [23].
Schnetzler et al. [24] estimated that about 6 million men in Europe could avoid the healthcare system to get PDE-5 medicines themselves. Market globalisation and the Internet are offering new scenarios and creating unknown risks for public health due to the growing attitude to buy drugs from the illegal market and online shops, exposing in this way more and more persons to the hazards due to the intake of illicit and counterfeit drugs [25]. Authorities developed specific legislative rules, particularly the European Directive 2011/62/UE, to avoid the trade of falsified medicinal products through the permitted supply chain. This Directive also normalises the Internet market of legal medicines by specifying that legal online pharmacies are obligated to exhibit a “common logo” on each page of the website dedicated to drugs sale [26][27]. An exciting and recent study [28] listed 80 new sexual performance enhancers detected illegally in the market that mimics the approved PDE-5 inhibitors.
3. Content of PDE-5 Inhibitors in WWTPs and STPs
Together with many other pharmaceutical products, these substances, once eliminated in faeces and urine, are transported through sewers to municipal wastewater treatment plants. Here, the parent substances and their metabolites are treated in biological reactors, which can only partially degrade them while triggering other transformation processes. They also often undergo accumulation processes before being discharged into the receiving water bodies. For this reason, investigations about the residues of these pharmaceuticals in the environment are essential to proceed with their removal. The first approach consists of determining the PDE-5 inhibitors’ content in untreated wastewater samples taken at the entry into WWTPs. Despite the low or very low solubility in water and the positive values of the partition coefficients (Log P), these products can arrive at municipal wastewater treatment plants adsorbed on the solid organic materials or dispersed in the liquid mass. Their lipophilicity favours their transport.
Research performed in eight WWTPs serving the catchment inside the towns of Bristol, Brussels, Castellón, Copenhagen, Milan, Oslo, Utrecht and Zurich [29] showed the presence of sildenafil and its two human urinary metabolites, desmethyl- and desethyl-sildenafil with amounts up to 60 ng L−1. They did not detect tadalafil and vardenafil in appreciable concentrations. The authors transformed the concentrations found in the collected samples to normalised loads and estimated the possible intake of sildenafil as amounts back-calculated from these loads. Moreover, they gathered the national prescription data from five countries in the form of the number of prescribed daily doses and transformed them into predicted loads for assessment. In Utrecht and Brussels, prescription data could only partially clarify the total quantity determined in wastewater.
In contrast, in Bristol, Milan and Oslo, the authors found that drug amounts in wastewater were lower than predicted from the prescription data. These studies illustrate the theoretical capacity of performed investigations to assess the use of imitating fraudulent medication and criminal online sales. Other researchers in Tarragona (Spain) and Germany determined the occurrence of these pharmaceuticals in WWTP influent and effluent water and sewage sludge [30] to evaluate the removal efficiency of the treatment systems and the possible influence of STPs on the pollution of the aquatic systems.
Sildenafil was the principal drug in all investigated water and sewage samples at a few ng L−1 and ng g−1 range, respectively. Tadalafil was not identified or below the limit of detection (LOD) in effluent water collected in Spain but was revealed in sewage sludge (12 ng g−1–LOD). Vardenafil was detected only in one sludge sample and between 5 ng g−1 and the LOD value in effluent water. The higher elimination efficiency of the STP in Tarragona (Spain) was 68%, 69% and 80% for sildenafil, tadalafil and vardenafil, respectively.
The monitoring evidenced the maximum concentrations for all drugs during the summer, probably due to the touristic fluxes (Figure 2, from [30]).
Similar quantities of these drugs were also detected by Schroeder et al. in a fitness centre discharge in Germany [31] and by Papageorgiou et al. in WWTPs in Volos, Greece [32]. Beyond the levels found for these substances, it is crucial to consider that the hazard to the environment may derive from their transformation compounds, as described by Temussi et al. [33] and Eichhorn et al. [34].
4. Removal Treatments
There are different methods able to remove several emerging contaminants such as photolysis, biological degradation, filtration on carbonised materials [35][36], catalytic use of green-synthesised copper nanoparticles (Cu NPs) [37] and adsorption on activated carbon [38].
4.1. Advanced Oxidation Treatment
Chlorination is by far the most adopted method in the disinfection stage in WWTPs. Wastewater is frequently added with chlorine as a sodium hypochlorite solution. However, the mixture HOCl/OCl−, recognised as free available chlorine, is a potent non-specific oxidant that promotes transformation reactions of many micro-pollutants. As a result, chlorinated and oxidised by-products are usually obtained, which could be more toxic than the parent substances [39].
4.2. Biological Degradation
De Felice et al. [40] studied the genetic outline of the microbial community emerging in a sildenafil-polluted aquatic environment. They isolated the 16S and 18S rRNA genes for bacteria and fungi, amplified by polymerase chain reaction (PCR) and separated using the denaturing gradient gel electrophoresis (DGGE) technique. Analysis of DGGE data indicated that the microbial composition changed with numerous major species in the last period of the experiment. This result suggests that great variety in microorganisms might be necessary for the effective biodegradation of pollutants. When more microbial species with different physiological capabilities are involved, many diverse organic pollutants might be degraded. In general, the use of mixed microbial consortia to remediate polluted sites is encouraged.
4.3. Adsorption on Activated Carbon
Delgado et al. [38] reported the efficiency of eliminating sildenafil citrate from water using powdered activated carbon. Having obtained a removal efficiency greater than 85%, they promoted the use of activated carbon as an effective tool for removing these kinds of recalcitrant emerging pollutants.
5. Toxicity Assessment
De Felice et al. [40] tested the biological degradation and the possible toxicity of microbial transformation substances of sildenafil in several living models such as Daphnia magna and Pseudokirchneriella subcapitata microalgae and human HepG2 cells to evaluate the mitochondrial activity.
Toxicological tests showed that only the initial sildenafil concentration (400 mg L−1) was toxic for D. magna and caused a decrease of cellular viability by 50% for HepG2 cells (Figure 1A,B, respectively). These assays revealed the absence of toxicity for the biotransformation products (Figure 1C).
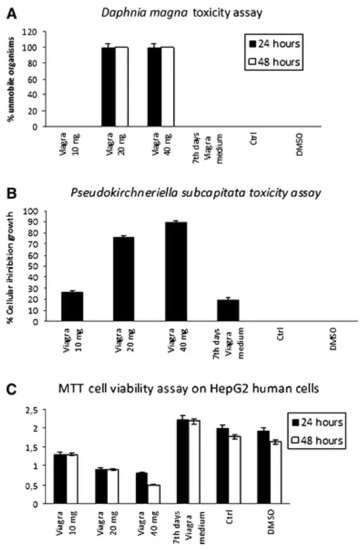
Figure 6. (A) Viability of Daphnia magna after 24 and 48 h incubation with sildenafil citrate (mean ± SD). (B) Viability of Pseudokirchneriella subcapitata after 24 h incubation with sildenafil citrate (mean ± SD). (C) Viability of HepG2 cells after 24 h incubation with sildenafil citrate (mean ± SD) [40]. Reprinted with permission from ref. [40]. Copyright 2021 Springer Nature.
Temussi et al. [33] carried out aquatic acute and chronic toxicity tests with Brachionus calyciflorus and Ceriodaphnia dubia and mutagenesis and genotoxicity analyses for some bacterial strains to evaluate the adverse environmental effects of parent compounds and transformation products obtained simulating the chlorination stage of a WWTP.
The authors selected the freshwater rotifer B. calyciflorus and the microcrustacean C. dubia as demonstrative aquatic organisms because they have an extensive geographic distribution and substantially impact water’s critical ecological processes. Furthermore, the authors performed mutagenesis and genotoxicity assays using the Ames test on Salmonella typhimurium and the SOS Chromotest on Escherichia coli PQ37 to detect the induction of point mutations of the SOS DNA repair system. The results of the acute toxicity tests are in Table 2.
Table 2. Acute LC50 values in mg L−1 with confidence limits (95% probability) of sildenafil and tadalafil and their respective derivatives S1 and T1 [33]. Reprinted with permission from ref. [33]. Copyright 2021 Elsevier.
| B. calyciflorus | C. dubia | |
|---|---|---|
| Sildenafil citrate | 42.74 (34.32–53.21) |
5.74 (3.08–10.71) |
| S1 | 19.82 (17.51–22.43) |
6.60 (5.73–7.60) |
| Tadalafil | NE up to 20 | NE up to 20 |
| T1 | NE up to 20 | NE up to 20 |
NE: no effect.
Chemical analysis revealed that the actual number of drugs diverged from the nominal one by less than 10%, so he researchers calculated the EC50 values using the nominal concentrations.
Tadalafil did not exhibit any acute consequence on both organisms up to the highest concentration tested (20 mg L−1). Sildenafil did not display any effect up to the highest concentration tested (10 mg L−1) for B. calyciflorus, while it showed an EC50 value of 0.64 mg L−1 for C. dubia. Chlorine derivatives of both drugs evidenced a different behaviour: the compound S1 showed long-term effects for both test organisms, while the product T1 only for crustaceans.
Literature on the additional biological effects of drugs such as mutagenesis and genotoxicity is also available [41][42][43]. The risk associated with small quantities of drugs is frequently due to their mutagenic and genotoxic potential already examined on mammalian cell lines or bacterial models. Sildenafil and tadalafil and the respective derivatives could not induce an SOS activation response, while the results of the Ames test were fascinating (Table 3). Tadalafil expressed a significant mutagenic activity (maximum MR = 18.4) in TA98 with a positive range of concentrations from 0.625 to 10 μg mL−1, and it was positive also in TA100 (2.5–10 μg mL−1). The derivative T1 had a mutagenic potential (2.5–10 μg mL−1) for TA98, but it was negative for TA100. Rocco et al. [44] studied the possible genetic damage of sildenafil through a Comet assay, diffusion assay and RAPD-PCR for the erythrocytes of Danio rerio. They found statistically significant genotoxicity.
Table 3. Ames test results for parent compounds, chlorine derivatives and positive and negative controls. In bold are the positive MRs [33]. Reprinted with permission from ref. [33]. Copyright 2021 Elsevier.
| Compounds | Ames Test | |||||
|---|---|---|---|---|---|---|
| TA98 | TA100 | |||||
| Concentration (μg mL−1) | Mean Revertants/Plate (±SD) | MR a | Concentration (μg mL−1) | Mean Revertants/Plate (±SD) | MR a | |
| Negative Control2-Nitrofluoren | - | 29.6 ± 2.7 | - | - - |
190.1 ± 47.2 - |
- - |
| 2.5 | 61.0 ± 5.7 | 2.1 | ||||
| 5 | 83.2 ± 11.3 | 2.8 | ||||
| 10 | 178.0 ± 15.0 | 6 | ||||
| Sodium azide | - | - | - | 5 | 488.0 ± 96.9 | 2.6 |
| 10 | 794.7 ± 90.9 | 4.2 | ||||
| 20 | 1153.3 ± 239.8 | 6.1 | ||||
| Sildenafil citrate | 0.625 | 46.7 ± 13.1 | 1.6 | 0.625 | 161.0 ± 18.7 | 0.8 |
| 1.25 | 48.7 ± 5.1 | 1.6 | 1.25 | 164.6 ± 8.7 | 0.9 | |
| 2.5 | 72.0 ± 11.3 | 2.4 | 2.5 | 190.6 ± 12.6 | 1 | |
| 5 | 93.0 ± 4.4 | 3.1 | 5 | 198.3 ± 24.0 | 1 | |
| 10 | 170.7 ± 32.0 | 5.8 | 10 | 220.0 ± 46.8 | 1.2 | |
| S1 | 0.3125 | 27.8 ± 3.2 | 0.9 | 0.3125 | 241.1 ± 27.5 | 1.3 |
| 0.625 | 37.6 ± 4.7 | 1.3 | 0.625 | 291.1 ± 21.6 | 1.5 | |
| 1.25 | 37.3 ± 11.6 | 1.3 | 1.25 | 303.1 ± 44.7 | 1.6 | |
| 2.5 | 53.6 ± 9.0 | 1.8 | 2.5 | 376.6 ± 45.3 | 2 | |
| 5 | 64.7 ± 20.2 | 2.2 | 5 | 405.1 ± 82.5 | 2.1 | |
| 10 | 112.4 ± 41.5 | 3.8 | 10 | 444.2 ± 83.2 | 2.3 | |
| Tadalafil | 0.3125 | 49.5 ± 7.7 | 1.7 | 0.3125 | 298.5 ± 38.7 | 1.6 |
| 0.625 | 67.2 ± 12.2 | 2.3 | 0.625 | 318.6 ± 65.2 | 1.7 | |
| 1.25 | 149.3 ± 33.2 | 5 | 1.25 | 325.6 ± 94.4 | 1.7 | |
| 2.5 | 223.0 ± 54.8 | 7.5 | 2.5 | 425.3 ± 80.9 | 2.2 | |
| 5 | 337.3 ± 32.3 | 11.4 | 5 | 556.0 ± 41.7 | 2.9 | |
| 10 | 544.0 ± 92.1 | 18.4 | 10 | 880.0 ± 237.6 | 4.6 | |
| T1 | 0.625 | 43.6 ± 11.9 | 1.5 | 0.625 | 286.0 ± 14.1 | 1.5 |
| 1.25 | 43.5 ± 14.0 | 1.5 | 1.25 | 310.7 ± 43.9 | 1.6 | |
| 2.5 | 85.4 ± 19.2 | 2.9 | 2.5 | 322.7 ± 28.4 | 1.7 | |
| 5 | 221.8 ± 57.2 | 7.5 | 5 | 288.0 ± 40.6 | 1.5 | |
| 10 | 391.2 ± 69.4 | 13.2 | 10 | 410.7 ± 70.0 | 2.2 | |
±SD = standard deviation obtained from three independent experiments. a MR (mutagenic ratio): number of revertants/plate compared to the negative control.
6. Impact on the Aquatic Organisms
To better understand the mechanisms that allow marine invertebrates to survive and reproduce in contaminated and changing habitats, researchers investigated the effect of some drugs, including sildenafil, on aquatic organisms. In particular, Zanuri et al. [45] studied Asterias rubens, Psammechinus miliaris and Arenicola marina (Polychaeta), essential components of marine benthos. They investigated the effects of exposure time and dosage of diclofenac, ibuprofen and sildenafil citrate on sperm motility and subsequent fertilisation. Diclofenac concentrations ≥ 0.1 μg L−1 caused a reduction in motility for all species observed. Exposure to ≥ 1.0 μg L−1 ibuprofen affected only P. miliaris gametes and A. marina fertilisation. Only the spermatozoa of A. rubens and P. miliaris exposed to sildenafil citrate at concentrations ≥ 18 and ≥ 50 ng L−1, respectively, showed higher percentage motility and a significant increase in fertilisation [45] (Figure 2; Table 4). Sildenafil citrate was non-toxic in all cases.
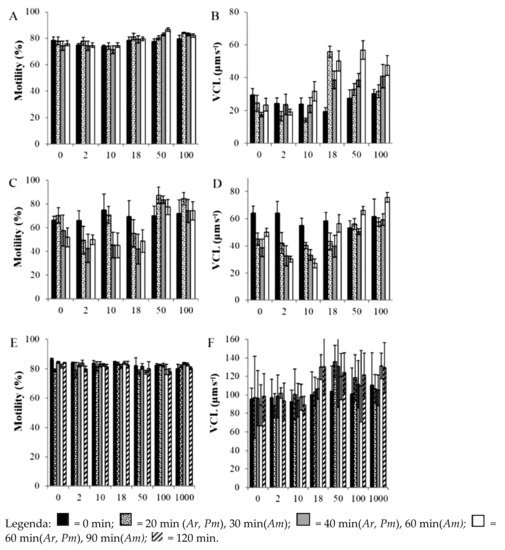
Figure 2. Percentage of sperm motility (A,C,E) and curvilinear velocity (VCL) (B,D,F) of sperm of Asterias rubens (A,B), Psammechinus miliaris (C,D), and Arenicola marina (E,F) after exposure to sildenafil citrate for set periods [45]. Reprinted with permission from ref. [45]. Copyright 2021 Elsevier.
Table 4. No-observed-effects (NOEC) or lowest-observed-effects concentration (LOEC), half-minimal effective concentration (EC50), and toxicity substance classification for Asterias rubens, Psammechinus miliaris and Arenicola marina. Toxicity classification is from EU Directive 93/67/EEC: EC50 (µg L−1) > 100,000 = non-toxic; 10,000–100,000 = harmful; 1000–10,000 = toxic; <100–1000 = very toxic; and <100 = extremely toxic. N/A = not applicable as an EC50 could not be calculated and is therefore deemed non-toxic [45]. Reprinted with permission from ref. [45]. Copyright 2021 Elsevier.
| Species | Test | Pharmaceutical | NOEC or LOEC (µg L−1) |
EC50 (µg L−1) | Classification |
|---|---|---|---|---|---|
| Asterias rubens | Sperm motility | Sildenafil citrate | NOEC = 0.18 | 60 min = 2.25 × 1012 | Non-toxic |
| Fertilisation: sperm pre-incubation | Sildenafil citrate | NOEC = 0.010 | 60 min = 7.15 × 1013 | Non-toxic | |
| Fertilisation: oocyte pre-incubation | Sildenafil citrate | NOEC = 0.10 | N/A | N/A | |
| Fertilisation: sperm and oocyte pre-incubation | Sildenafil citrate | NOEC = 0.01 | 60 min = 2.37 × 1012 | Non-toxic | |
| Psammechinus miliaris | Sperm motility | Diclofenac Ibuprofen Sildenafil citrate |
NOEC = 0.01 NOEC = 0.1 NOEC = 0.018 |
60 min = 378.22 60 min = 845.98 60 min = 7.23 × 1010 |
Very toxic Very toxic Non-toxic |
| Fertilisation: sperm pre-incubation | Sildenafil citrate | NOEC = 0.10 | 60 min = 6.241 × 1010 | Non-toxic | |
| Fertilisation: oocyte pre-incubation | Sildenafil citrate | NOEC = 0.01 | N/A | N/A | |
| Fertilisation: sperm and oocyte pre-incubation | Diclofenac Ibuprofen Sildenafil citrate |
LOEC = 0.01 NOEC = 0.10 NOEC = 1.0 |
60 min = 247.31 60 min = 792.96 N/A |
Very toxic Very toxic N/A |
|
| Arenicola marina | Sperm motility | Sildenafil citrate | NOEC = 1.0 | N/A | N/A |
| Fertilisation: sperm pre-incubation | Diclofenac Ibuprofen Sildenafil citrate |
NOEC = 1.00 NOEC = 0.10 NOEC = 1.0 |
120 min = 565.53 120 min = 3.24 × 109N/A |
Very toxic Non toxic N/A |
|
| Fertilisation: oocyte pre-incubation | Sildenafil citrate | NOEC = 1.00 | N/A | N/A | |
| Fertilisation: sperm and oocyte pre-incubation | Sildenafil citrate | NOEC = 1.0 | N/A | N/A |
7. Conclusions and Future Perspectives
The potential effects of PDE-5 inhibitors on the environment are not still apparent. However, ED medicines will prove to have very high usage rates in the near future, mainly because they are very diffuse and popular drugs. The availability of EDs without prescription over the Internet and their use for ludic activity can yield an increased potential for environmental exposure and conceivable non-target effects.
Risk is connected principally to the accumulation, persistence and toxicity of pharmaceutical compounds diffused in the environment rather than the actual quantity released. Given their bioactive nature, even small amounts of this kind of drug could impact delicate organisms; in addition, synergic action with other contaminants might have additive effects once released into the environment, especially if they insist on the same receptors of biological organisms.
Since these substances are rapidly becoming one of the most widespread and extensively used drugs at the world level, improper disposal in the environment is a reason of general concern.
More detailed knowledge of biotic and abiotic processes, able to interfere with these pollutants, will notably improve the used remediation strategies.
This entry is adapted from the peer-reviewed paper 10.3390/w13202859
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65.
- Tran, N.H.; Reinhard, M.; Yew-Hoong Gin, K. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2017, 133, 182–207.
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of therapeutic drugs in the environment. Lancet 2000, 355, 1789–1790.
- Susa, S.T.; Preuss, C.V. Drug Metabolism. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lützhøft, H.C.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393.
- Zhou, J.; Broodbank, N. Sediment-water interactions of pharmaceutical residues in the river environment. Water Res. 2014, 48, 61–70.
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363.
- Buser, H.R.; Müller, M.D.; Theobald, N. Occurrence of the pharmaceutical drug clofibric acid and the herbicide mecoprop in various Swiss Lakes and in the North Sea. Environ. Sci. Technol. 1998, 32, 188–192.
- Calamari, D.; Zuccato, E.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Strategic survey of therapeutic drugs in the rivers Po and Lambro in Northern Italy. Environ. Sci. Technol. 2003, 37, 1241–1248.
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580.
- Hernández, A.F.; Gil, F.; Lacasaña, M. Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223.
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 1092–2172.
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitor. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021; Available online: http://www.ncbi.nlm.nih.gov/books/nbk549843/ (accessed on 2 September 2021).
- Ghofrani, H.A.; Osterloh, I.H.; Grimminger, F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat. Rev. Drug Discov. 2006, 5, 689–702.
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. Pharm. Ther. 2013, 38, 407–419. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3776492/ (accessed on 2 September 2021).
- Nichols, D.J.; Muirhead, G.J.; Harness, J.A. Pharmacokinetics of sildenafil citrate after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. Suppl. 2002, 53, 5S–12S.
- Stark, S.; Sachse, R.; Liedl, T.; Hensen, J.; Rohde, G.; Wensing, G.; Horstmann, R.; Schrott, K.M. Vardenafil increases penile rigidity and tumescence in men with erectile dysfunction after a single oral dose. Eur. Urol. 2001, 40, 181–190.
- Jung, J.; Choi, S.; Cho, S.H.; Ghim, J.L.; Hwang, A.; Kim, U.; Kim, B.S.; Koguchi, A.; Miyoshi, S.; Okabe, H.; et al. Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: A single- and multiple-dose, double-blind, randomised, placebo-controlled, dose-escalation study in healthy Korean male volunteers. Clin. Ther. 2010, 32, 1178–1187.
- Patterson, B.; Bedding, A.; Jewelll, H.; Payne, C.; Mitchell, M. The effect of intrinsic and extrinsic factors on the pharmacokinetic properties of tadalafil (IC351). Int. J. Impot. Res. 2001, 13, S62.
- Pfizer Labs. VIAGRA (Sildenafil Citrate); Pfizer: New York, NY, USA, 2010.
- Bayer HealthCare. LEVITRA (Vardenafil HCl); Bayer Pharmaceuticals Corporation: West Haven, CT, USA, 2014.
- Vivus. STENDRA (Avanafil); Vivus: Campbell, CA, USA, 2012.
- Shim, Y.S.; Pae, C.U.; Cho, K.J.; Kim, S.W.; Kim, J.C.; Koh, J.S. Effects of daily low-dose treatment with phosphodiesterase type 5 inhibitor on cognition, depression, somatisation and erectile function in patients with erectile dysfunction: A double-blind, placebo-controlled study. Int. J. Impot. Res. 2014, 26, 76–80.
- Schnetzler, G.; Banks, I.; Kirby, M.; Zou, K.H.; Symonds, T. Characteristics, behaviors, and attitudes of men bypassing the healthcare system when obtaining phosphodiesterase type 5 inhibitors. J. Sex. Med. 2010, 7, 1237–1246.
- Gaudiano, M.C.; Manna, L.; Rodomonte, A.L.; Bartolomei, M.; Bertocchi, P.; Gallinella, B.; Antoniella, E.; Muleri, N.; Civitelli, G.; Alimonti, S.; et al. A Survey on Illegal and Counterfeit Medicines for the Treatment of Erectile Dysfunctions in Italy. J. Sex. Med. 2012, 9, 2130–2137.
- Mustazza, C.; Gaudiano, M.C.; Borioni, A.; Valvo, L. The evolution of the illegal market of falsified medicines and the experience of the Italian OMCL: From control to research. Ann. Dell’istituto Super. Sanita 2018, 54, 267–269.
- European Union. Directive 2011/62/EU of the European Parliament and of the Council of 8 June 2011 Amending Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use; European Union: Brussels, Belgium, 2011.
- Kee, C.L.; Ge, X.; Gilard, V.; Malet-Martino, M.; Low, M.Y. A review of synthetic phosphodiesterase type 5 inhibitors (PDE-5i) found as adulterants in dietary supplements. J. Pharm. Biomed. Anal. 2018, 147, 250–277.
- Causanilles, A.; Rojas Cantillano, D.; Emke, E.; Bade, R.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Gracia-Lor, E.; Hernández, F.; Kasprzyk-Hordern, B.; et al. comparison of phosphodiesterase type V inhibitors use in eight European cities through analysis of urban wastewater. Environ. Int. 2018, 115, 279–284.
- Nieto, A.; Peschka, M.; Borrull, F.; Pocurull, E.; Marcé, R.M.; Knepper, T.P. Phosphodiesterase type V inhibitors: Occurrence and fate in wastewater and sewage sludge. Water Res. 2010, 44, 1607–1615.
- Schröder, H.F.; Gebhardt, W.; Thevis, M. Anabolic, doping, and lifestyle drugs, and selected metabolites in wastewater-detection, quantification, and behaviour monitored by high-resolution MS and MS n before and after sewage treatment. Anal. Bioanal. Chem. 2010, 398, 1207–1229.
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569.
- Temussi, F.; DellaGreca, M.; Pistillo, P.; Previtera, L.; Zarrelli, A.; Criscuolo, E.; Lavorgna, M.; Russo, C.; Isidori, M. Sildenafil and tadalafil in simulated chlorination conditions: Ecotoxicity of drugs and their derivatives. Sci. Total Environ. 2013, 463, 366–373.
- Eichhorn, P.; Pérez, S.; Aceña, J.; Gardinali, P.; Abad, J.L.; Barceló, D. Identification of phototransformation products of sildenafil (Viagra) and its N-demethylated human metabolite under simulated sunlight. J. Mass Spectrom. 2012, 47, 701–711.
- Coimbra, R.N.; Calisto, V.; Ferreira, C.I.A.; Esteves, V.I.; Otero, M. Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolysed pulp mill sludge. Arab. J. Chem. 2019, 12, 3611–3620.
- Jaria, G.; Calisto, V.; Silva, C.P.; Gil, M.V.; Otero, M.; Esteves, V.I. Obtaining granular activated carbon from paper mill sludge—A challenge for application in the removal of pharmaceuticals from wastewater. Sci. Total Environ. 2019, 653, 393–400.
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339.
- Delgado, N.; Capparelli, A.; Navarro, A.; Marino, D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manag. 2019, 236, 301–308.
- Escher, B.I.; Fenner, K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 2011, 45, 3835–3847.
- De Felice, B.; Argenziano, C.; Guida, M.; Trifuoggi, M.; Russo, F.; Condorelli, V.; Inglese, M. Molecular characterisation of microbial population dynamics during sildenafil citrate degradation. Mol. Biotechnol. 2009, 41, 123–132.
- Isidori, M.; Nardelli, A.; Pascarella, L.; Rubino, M.; Parrella, A. Toxic and genotoxic impact of fibrates and their photoproducts on non-target organisms. Environ. Int. 2007, 33, 635–641.
- Isidori, M.; Parrella, A.; Pistillo, P.; Temussi, F. Effects of ranitidine and its photoderivatives in the aquatic environment. Environ. Int. 2009, 35, 821–825.
- Vasquez, M.I.; Garcia-Käufer, M.; Hapeshi, E.; Menz, J.; Kostarelos, K.; Fatta-Kassinos, D.; Kümmerer, K. Chronic ecotoxic effects to Pseudomonas putida and Vibrio fischeri, and cytostatic and genotoxic effects to the hepatoma cell line (HepG2) of ofloxacin photo(cata)lytically treated solutions. Sci. Total Environ. 2013, 450, 356–365.
- Rocco, L.; Frenzilli, G.; Zito, G.; Archimandritis, A.; Peluso, C.; Stingo, V. Genotoxic effects in fish induced by pharmacological agents present in the sewage of some Italian water-treatment plants. Environ. Toxicol. 2012, 27, 18–25.
- Zanuri, N.B.M.; Bentley, M.G.; Caldwell, G.S. Assessing the impact of diclofenac, ibuprofen and sildenafil citrate (Viagra®) on the fertilisation biology of broadcast spawning marine invertebrates. Mar. Environ. Res. 2017, 127, 126–136.
This entry is offline, you can click here to edit this entry!
