To achieve curative resection for pancreatic cancer during pancreaticoduodenectomy (PD), extensive portal vein (PV) resection, including porto-mesenterico-splenic confluence (PMSC), may sometimes be necessary if the tumor is close to the portal venous system. Recently, this extended resection has been widely accepted in high-volume centers for pancreatic resection due to its favorable outcomes compared with non-operative treatment. However, in patients with long-term survival, sinistral portal hypertension (SPH) occurs as a late-onset postoperative complication. These patients present gastrointestinal varices due to congested venous flow from the spleen, which may cause critical variceal bleeding.
1. Introduction
Sinistral (left-sided) portal hypertension (SPH) was originally reported by Turrill et al. in 1969 as gastroesophageal variceal bleeding resulting from the splenic vein (SV) occlusion [
1]. This symptom has been well reported since the 1900s, and a review article in 1970 summarized the etiology of isolated SV occlusion as follows: neoplasia, pancreatitis, trauma, pseudocyst, infection, miscellaneous, and unknown [
2]. Thereafter, Evans [
3] defined “Sinistral (left-sided) portal hypertension” as a clinical syndrome of SV thrombosis caused by pancreatic pathology which manifests as bleeding in the gastric varices in patients with a patent portal vein (PV) and normal hepatic function.
The same symptoms were observed in patients who underwent pancreaticoduodenectomy (PD) with SV resection. Fortner et al. reported symptoms such as hemorrhagic stomach, an enlargement of the spleen, or spontaneous splenic rupture during regional subtotal pancreatectomy with SV ligation as a result of venous congestion [
4]. They also commented that such congestion was a rare occurrence, although a splenectomy was necessary during surgery in some cases. Since then, several reports have been published regarding the occurrence of gastrointestinal varices and bleeding after PD with porto-mesenterico-splenic confluence (PMSC) resection [
5,
6,
7,
8,
9]. However, due to the poor survival rate of patients with pancreatic ductal adenocarcinoma (PDAC) who underwent PV resection, reports of coherent cases are limited. In recent years, the prognosis of PDAC has gradually improved due to the progress of multidisciplinary therapies, including chemotherapy and radiotherapy, and accordingly, the number of reports on SPH has been increasing over the past decade.
The definition of SPH after PD varies among reports. The occurrence of bleeding from varicose veins after SV ligation/resection without liver disease or PV stenosis/occlusion would correspond to the original definition [
3,
10], however, the incidence of varicose veins, the enlargement of the spleen, thrombocytopenia, and persistent abdominal pain were also listed in the previous study as defining features of SPH [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. It should be noted that the direct cause of gastrointestinal bleeding is varicose vein formation and patients with varicose veins are at risk of gastrointestinal bleeding in the future. Postoperative spleen hypertrophy, thrombocytopenia, and abdominal pain may result from the high SV pressure or splenomegaly, but these could also be induced by tumor recurrence, PV stenosis, liver disease, chemotherapy, or various drugs.
Cases without SV resection were previously compared to those with SV resection, and SV resection was proven to increase variceal formation or variceal bleeding [
13,
21]. However, it can be difficult to preserve SV during PD if the tumor is located close to the PMSC or if tumor invasion is suspected; therefore, a thorough understanding of SPH is necessary for pancreatic surgeons.
2. Pathogenesis of SPH
To investigate the pathogenesis of SPH following PD, it is necessary to understand the drainage routes from the spleen. There are two distinct pathways for these drainage routes: systemic circulation and PV circulation. The former is a spontaneous splenorenal shunt. This physiological collateral is usually evident postoperatively and occurs in approximately 10% of patients with SV ligation [
11]. Spontaneous spleno-adreno-renal shunt and other collateral routes draining into systemic circulation via the retroperitoneal venous system or esophageal submucosa are rare but may develop after PD with PMSC resection [
22].
For the latter case, Strasberg et al. introduced two major pathways: the superior route and the inferior route [
10,
15]. They claimed that the superior route passed from the SV around and through the stomach to enter the PV via the left gastric vein. This route has been previously reported as the classical SPH venous pathway [
2,
23] and was also stressed as an important SV drainage route to prevent SPH after PD with PMSC resection [
14] (
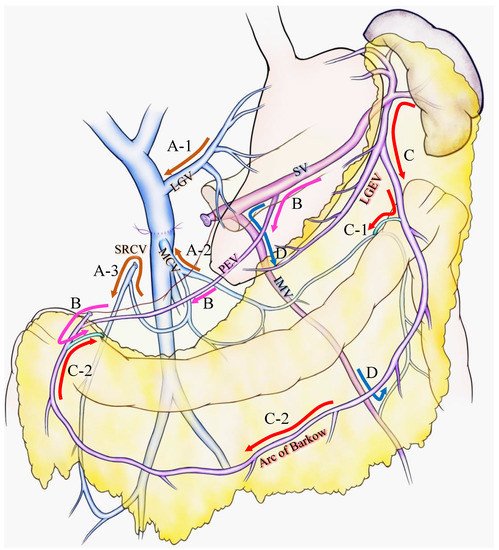
Figure 1A-1). The inferior route passed from the SV through the root of the mesentery (the posterior epiploic vein) (
Figure 1B), the omental arcade (arc of Barlow), or the colonic vein to the superior mesenteric vein (SMV) (
Figure 1C). Strasberg et al. also emphasized the importance of a longer length of residual SV to preserve the posterior epiploic vein or the left gastric epiploic vein, which sometimes flow into the SV in the pancreas and develop as a collateral vein.
Figure 1. Various venous flow form spleen after portal-superior mesenteric vein confluence resection. Brown arrows indicate the critical veins: (A-1) LGV, (A-2) MCV, (A-3) and SRCV arcade. (B) Pink arrows indicate the route from the SV to the PEV and the colonic marginal vein. (C) Red arrows indicate the route from the SV to the LGEV and the arc of Barkow; (C-1) the connection between the arc of Barkow and the colonic marginal vein at the left side of the transverse colon; (C-2) the connection between the arc of Barkow and the colonic marginal vein at the right side of the transverse colon. (D) Blue arrows indicate the route from the SV to the IMV and colonic marginal vein. IMV: inferior mesenteric vein; LEGV: left gastric epiploic vein; LGV: left gastric vein; MCV: middle colic vein; PEV: posterior epiploic vein; SV: splenic vein; SRCV: superior right colic vein.
Our group has confirmed that these superior and inferior routes [
11,
17] reported a high incidence (62.8%) of colonic varices, if both the left gastric vein (LGV) and middle colic vein (MCV) were sacrificed during PD with SV ligation [
17]. We emphasized the importance of the superior right colic vein (SRCV) arcade to prevent variceal development at the right flexure of the colon (
Figure 1A-3). Thereafter, the LGV, MCV, and the SRCV arcade were defined as the critical veins [
11] (
Figure 1A), in which LGV corresponds to the superior route (
Figure 1A-1), and the MCV and SRCV arcade correspond to the inferior route (
Figure 1A-2,3).
A splenic vein–inferior mesenteric vein (SV-IMV) anastomosis or the preservation of a natural SV-IMV confluence has been emphasized to prevent SPH [
7,
14,
18,
19,
24,
25], whereas some recent reports indicate that inferior mesenteric vein (IMV) preservation was not related to the incidence of SPH [
10,
11,
13,
15,
17]. Pligrim et al. reported three representative cases of the relationship between gastrointestinal bleeding and the SV-IMV junction. In their report, two patients experienced gastrointestinal bleeding despite the presence of a patent SV-IMV in one patient [
19]. Our group and Mizuno et al. suggested that the preservation of the IMV was not associated with SPH in their reports [
13,
17]. Rosado et al. divided IMV into 15 cases without any occurrence of SPH [
15]. As shown in
Figure 1D, IMV could be a promising route to bridge the SV to the colonic marginal vein, although it could be replaced by other collaterals, including the arc of Barkow, which connects SV to the colonic marginal vein (
Figure 1C). In addition, the IMV sometimes fails to connect with the SMV due to an incomplete colonic venous arcade, which could be one reason as to why the preservation of IMV is not related to SPH occurrence. Thus, IMV is not found to be a critical vein for preventing SPH. However, given that the communication between the arc of Barkow and the colonic marginal vein usually forms postoperatively, there is no guarantee that an adequate connection will develop after surgery; therefore, it may be important to maintain the IMV-SV junction to preserve the collateral route as best as possible if the oncologic goal of the operation can be achieved.
As a result of less superior or inferior routes after PD with PMSC resection, gastrointestinal varices develop in various intestinal regions. Four types of varicose veins were identified: colonic varices, pancreatojejunostomy varices, esophageal varices, and gastrojejunostomy varices [
17]. Varicose veins could be created in sites of the abdomen other than those mentioned above (such as rectal varices or varicose veins at the left side of the colon) due to diversity in the collateral route development. Our group reported that the number of remaining critical veins was inversely proportional to the incidence of variceal formation: 0 critical vein, 100% varices, 1 critical vein, 24% varices, 2 or more critical veins, and 0%. Thus, the pathogenesis of SPH is complicated because of the complex hemodynamics of collaterals after PD with PMSC resection.
Some studies indicated no gastrointestinal bleeding even after the incidence of varicose formation [
10,
14,
18,
21,
26,
27,
28], but others reported the incidence of severe variceal bleeding [
4,
5,
6,
7,
8,
9,
11,
13,
17,
19,
24,
29,
30,
31,
32,
33,
34,
35,
36]. This difference may be due to the dedicated adjustment of SV pressure by collateral routes from the spleen. Since gastrointestinal bleeding cannot occur without variceal formation, the development of gastrointestinal varices is the most important factor for SPH, which is strongly influenced by the number of remaining collaterals draining from the spleen [
11,
15]. Another important factor for gastrointestinal bleeding is SV pressure. Even after the development of variceal formation, 90% of patients did not experience gastrointestinal bleeding (
Table 1). This is because varicose veins alone are not a definitive cause of SPH, and the risk of bleeding seems to be affected by increased SV pressure. The volume of the spleen in patients with SV resection significantly increased 6 or 12 months after surgery compared to before surgery [
13,
14,
16,
17,
37], and in half of the patients with gastrointestinal bleeding, the spleen volume was doubled or greater compared with preoperative levels [
13,
17]. Reflecting the increased spleen volume after surgery, the platelet count ratio at 6 months after surgery in the patients after the SV resection was significantly lower than that in patients without the SV resection [
13,
16,
37]. This SV pressure could be controlled by increasing collaterals from the spleen, through methods such as SV reconstruction [
12,
13,
14,
15,
31,
38] or decreasing the blood inflow to the spleen (splenic artery ligation) [
16,
37].
Table 1. Frequency of variceal formation and gastrointestinal bleeding after PD with SV resection/ligation.
| No |
Author |
Journal |
Year |
Case No. of SV Resection |
N |
Varices |
Type of Evaluated Varices |
Bleeding |
| Esophageal |
Gastric |
Pancreatic |
Colonic |
(% of Varices) |
| 1 |
Ono et al. [17] |
Br. J. Surg. |
2015 |
Total cases (LGV−, MCV−) |
43 |
27 (62.8%) |
✔ |
✔ |
✔ |
✔ |
3 (11.1%) |
| 2 |
Hattori et al. [18] |
Dig. Surg. |
2015 |
Total cases |
81 |
7/31 (22.6%) * |
✔ |
✔ |
- |
- |
1 (14.3%) |
| 3 |
Gyoten et al. [16] |
World J. Surg. |
2016 |
Total cases |
72 |
44 (61.1%) |
✔ |
✔ |
✔ |
✔ |
4 (9.0%) |
| |
|
|
|
SAR− |
58 |
39 (67.2%) |
|
|
|
|
4 (10.3%) |
| |
|
|
|
SAR+ |
14 |
5 (35.7%) |
|
|
|
|
0 |
| 4 |
Rosado et al. [15] |
J. Gastrointest Surg. |
2017 |
Total cases (IMV−) |
15 |
3 (20.0%) |
✔ |
✔ |
- |
✔ |
0 |
| 5 |
Tanaka H et al. [14] |
HPB (Oxford) |
2017 |
Total cases |
29 |
3 (10.3%) |
✔ |
✔ |
- |
- |
0 |
| |
|
|
|
LGV preservation+ |
11 |
0 |
|
|
|
|
0 |
| |
|
|
|
LGV preservation− |
18 |
3 (16.7%) |
|
|
|
|
0 |
| 6 |
Tanaka M et al. [11] |
Surgery |
2019 |
Total cases ** |
88 |
41 (46.6%) |
✔ |
✔ |
✔ |
✔ |
5 (12.2%) |
| |
|
|
|
Critical vein: 0 |
29 |
29 (100%) |
|
|
|
|
4 (13.8%) |
| |
|
|
|
Critical vein: 1 |
51 |
12 (23.5%) |
|
|
|
|
1 (8.3%) |
| |
|
|
|
Critical veins: ≥ 2 |
8 |
0 |
|
|
|
|
0 |
| 7 |
Mizuno et al. [13] |
Ann. Surg. |
2019 |
Total cases |
251 |
93 (37.1%) |
✔ |
✔ |
✔ |
✔ |
10 (11.0%) |
| |
|
|
|
SAR−/SV reconstruction- |
227 |
84 (37.0%) |
|
|
|
|
9 (10.7%) |
| |
|
|
|
SAR+ |
12 |
4 (33.3%) |
|
|
|
|
0 |
| |
|
|
|
SV reconstruction+ |
12 |
5 (41.6%) |
|
|
|
|
1 (20%) |
| 8 |
Addeo et al. [31] |
Surgery |
2020 |
Total cases |
114 |
68 (59.6%) |
✔ |
✔ |
- |
✔ |
1 (1.5%) |
| |
|
|
|
SV reconstruction-(IMV−) |
36 |
29 (80.6%) |
|
|
|
|
1 (3.4%) |
| |
|
|
|
SV reconstruction+ |
78 |
39 (50%) |
|
|
|
|
0 |
| 9 |
Shiihara et al. [21] |
Pancreatology |
2020 |
Total cases |
36 |
20 (55.6%) |
✔ |
✔ |
✔ |
✔ |
0 |
| |
|
|
|
LGV preserved via PV |
8 |
0 |
|
|
|
|
0 |
| |
|
|
|
IMV preserved (LGV−) |
8 |
2 (25%) |
|
|
|
|
0 |
| |
|
|
|
LGV−, IMV− |
20 |
18 (90%) |
|
|
|
|
0 |
| 10 |
Yamada et al. [37] |
Langenbecks Arch. Surg. |
2021 |
Total cases |
63 |
16 (25.4%) |
✔ |
✔ |
✔ |
✔ |
NA *** |
| |
|
|
|
SAR− |
21 |
10 (47.6%) |
|
|
|
|
NA *** |
| |
|
|
|
SAR+ |
42 |
6 (14.3%) |
|
|
|
|
0 |
3. Frequency of Variceal Formation and Gastrointestinal Bleeding
Several reports from single centers indicated the incidence of SPH after PD with SV resection, and few reports surveyed the incidence in multiple centers. To investigate the frequency of variceal formation and gastrointestinal bleeding after PD with SV resection/ligation, all the studies that included more than 10 cases of PD with PMSC resection and information of varices and gastrointestinal bleeding are summarized in
Table 1 [
11,
13,
14,
15,
16,
17,
18,
21,
31,
37]. Technical studies such as “How I do it” were excluded from the analyses. In total, 10 studies were obtained, two of which were multicenter studies [
13,
37]. Some included overlapping data because they were reported in the same institution or multiple institutions, but were preserved in the table because they had different study concepts or included additional cases or findings. Multidetector enhanced computed tomography was used to detect gastrointestinal varices in all the studies and the endoscopic findings were included in some studies [
14,
17,
18,
21].
The frequency of varices ranged from 10.3% to 62.8% [
11,
13,
14,
15,
16,
17,
18,
21,
31,
37]. Although some of the authors reported a low incidence of variceal formation [
14,
15,
18], the definition of varicose veins varied from study to study. Most of the studies evaluated esophageal, gastric, pancreatic, and colonic varices; however, a few of the studies did not include pancreatic or colonic varices (
Table 1). Hattori and Tanaka H et al. excluded colonic varices from their analysis; consequently, the incidence of varices was relatively low, ranging from 10.3% to 22.6%. Conversely, Rosado et al. [
15] evaluated all types of varicose veins but reported a low incidence of varicose veins (20%) in their study, at a 1/3 of the rate reported by our group [
17]. To explain this difference, S. M. Strasberg personally contacted A. Saiura and noted, in the discussion [
15], that they preserved the whole greater omentum for the left-to-right omental venous channels (
Figure 1C), while our group resected the portion of the right side of the greater omentum (
Figure 1C-2), which resulted in a high incidence of right colonic varices. Aside from in the studies with a low incidence of varicose veins, the incidence of variceal formation has been reported to range from 37% to 62.8%, but this percentage is highly dependent on the number of preserved collateral veins or other factors, as shown in the subgroup analysis of each report (
Table 1).
The rate of gastrointestinal bleeding was approximately 10% in cases of varicose vein formation [
11,
13,
16,
17], although Addeo et al. [
31] reported a low incidence of gastrointestinal bleeding, for which the dilated collateral veins were included as varices, meaning that they may have overestimated the incidence of variceal formation [
31]. Gastrointestinal bleeding was not reported in any of the cases after PD with splenic artery ligation. The reason for this may be that the SV pressure was well-controlled in these cases, and the low SV pressure decreased the risk of gastrointestinal bleeding, even after varicose vein formation. Importantly, bleeding from gastrointestinal varices is categorized as a late-onset postoperative complication. Mizuno et al. summarized 10 cases of bleeding, occurring at a median of 20 months (8–99 months), postoperatively. As a result of the improved prognosis of PDAC through multidisciplinary treatment, more patients may experience SPH in the future.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13215334