Myocardium Infarction (MI) is one of the foremost cardiovascular diseases (CVDs) causing death worldwide, and its case numbers are expected to continuously increase in the coming years. Pharmacological interventions have not been at the forefront in ameliorating MI-related morbidity and mortality. Stem cell-based tissue engineering approaches have been extensively explored for their regenerative potential in the infarcted myocardium. Recent studies on microfluidic devices employing stem cells under laboratory set-up have revealed meticulous events pertaining to the pathophysiology of MI occurring at the infarcted site. This discovery also underpins the appropriate conditions in the niche for differentiating stem cells into mature cardiomyocyte-like cells and leads to engineering of the scaffold via mimicking of native cardiac physiological conditions. However, the mode of stem cell-loaded engineered scaffolds delivered to the site of infarction is still a challenging mission, and yet to be translated to the clinical setting.
- myocardial infarction
- stem cells
- regeneration
- biomaterial
- cardiomyocytes
- tissue engineering
1. Introduction
Cardiovascular disease, predominantly MI, is attributed the highest mortality rate worldwide [1]. Reduced contractility and function, irregular left ventricle remodeling, and uneven stress distribution in the heart muscle are among the complications occurring post-MI, eventually resulting in catastrophic heart failure. According to the American Heart Association (AHA)’s “Heart Disease and Stroke Statistics—2021”, the prevalence of CVD (including heart failure, hypertension, and stroke) in the US population is 49.2% in the age range of 20 years and above [2]. In 2014, 150,000 people died due to MI; thus an estimated approximately 14% of global death occurs mainly due to MI. Furthermore, MI survivors are also 15 times more likely to develop post-disease complications that lead to heart failure, and are prone to die sooner rather than later compared to the normal population [1]. Cardiac ischemia-related deaths have also ascended to the top of the list of causes of death in India, the United States, and Europe, apart from MI [1][3][4]. After MI incidence, male and female patients above 45 years of age have a lower life expectancy, of 8.2 and 5.5 years, respectively [1]. Socioeconomic burdens such as health care infrastructure and treatment costs (USD 11.5 billion) have made MI one of the top ten most expensive illnesses in the United States [1][5]. Researchers and clinicians around the world have been working extensively to reduce the global incidence of MI and develop significant cost-effective treatment strategies to reduce the mortality rate from MI.
The human heart is a complex organ composed of various types of cells such as cardiomyocytes (CM), fibroblasts, endothelial cells, valve interstitial cells, and resident cardiac stem cells. The cells of the heart are very active metabolically, as it physiologically requires adenosine tri-phosphate (ATP) for its function. Nonetheless, the heart lacks endogenous repair or regeneration potential, thus it remains devoid of regenerative capacity. Any defect in size or deficiency in cardiomyocyte numbers leads to life-threatening MI-related cardiovascular complications [6]. Currently, mitigation of CVDs by pharmaceutical drugs and other clinical practices have effectively improved the patient’s survival and quality of life after tissue damage [7]. However, this remains only a short-term solution of temporary duration; the permanent curative would be via heart transplant. Severe shortage of donor organs, post-graft complications, and the limited efficacy of pharmacological interventions has placed the emphasis on cell-loaded scaffold-based therapeutic approaches for cardiovascular complications (CVDs).
The emergence of cardiac tissue engineering (CTE) has not only given substantial hope for resolving or rescuing the damaged heart after MI but also for prompting the regeneration of the damaged myocardium, thus providing a permanent curative. The idea of CTE was first impelled in 1995 by in vitro-generated cardiac tissue obtained from embryonic chicken CMs. This further ushered in the prospect of new research areas around CTE, mainly idealised to translate the bench to bedside. CTE primarily aims to recapitulate the in vivo cardiac niche under in vitro conditions. Therefore, the long-term goals of CTE are considered the construction of in vitro-fabricated tissues for in vivo cardiac repair and regeneration, in vitro preclinical models for evaluation of drug toxicity, and disease models for understanding the development and pathophysiology of heart-related disorders [8]. With the global rise in CVD cases, it is essential to reinforce the treatment modalities for better disease management. In the current scenario, the previously mentioned CTE is considered as at the forefront; however, the conducting of numerous clinical trials is crucial in prioritizing CTE in clinical practice.
Delivery of an engineered scaffold loaded with stem cells, CM mitogens, or pharmacological molecules directly to the infarcted site via either the intra-coronary or intra-myocardial mode leads to the relatively prompt recovery of the infarcted tissue, followed by regeneration and regaining of functional significance. Still to be addressed are the current roadblocks to successive clinical utilization, such as an optimized protocol for stem cell-derived CMs and their source, biomaterials for CM cultures, as well as their delivery strategies [9]. Advanced delivery approaches using injectable or patch-based methods are recently gaining significant attention due to their complexity in design and versatility in application. The most advanced technology, using iPSC-derived CM-loaded microfluidic devices, has now been providing unprecedented opportunities to understand the mechanisms of MI development. This technology can also be employed to study the effects of drugs in the preclinical drug screening phase [10].
The recent technological advances in cardiac tissue engineering, for example, cell-based therapy and patch-based therapy, have been elucidated in Figure 1.
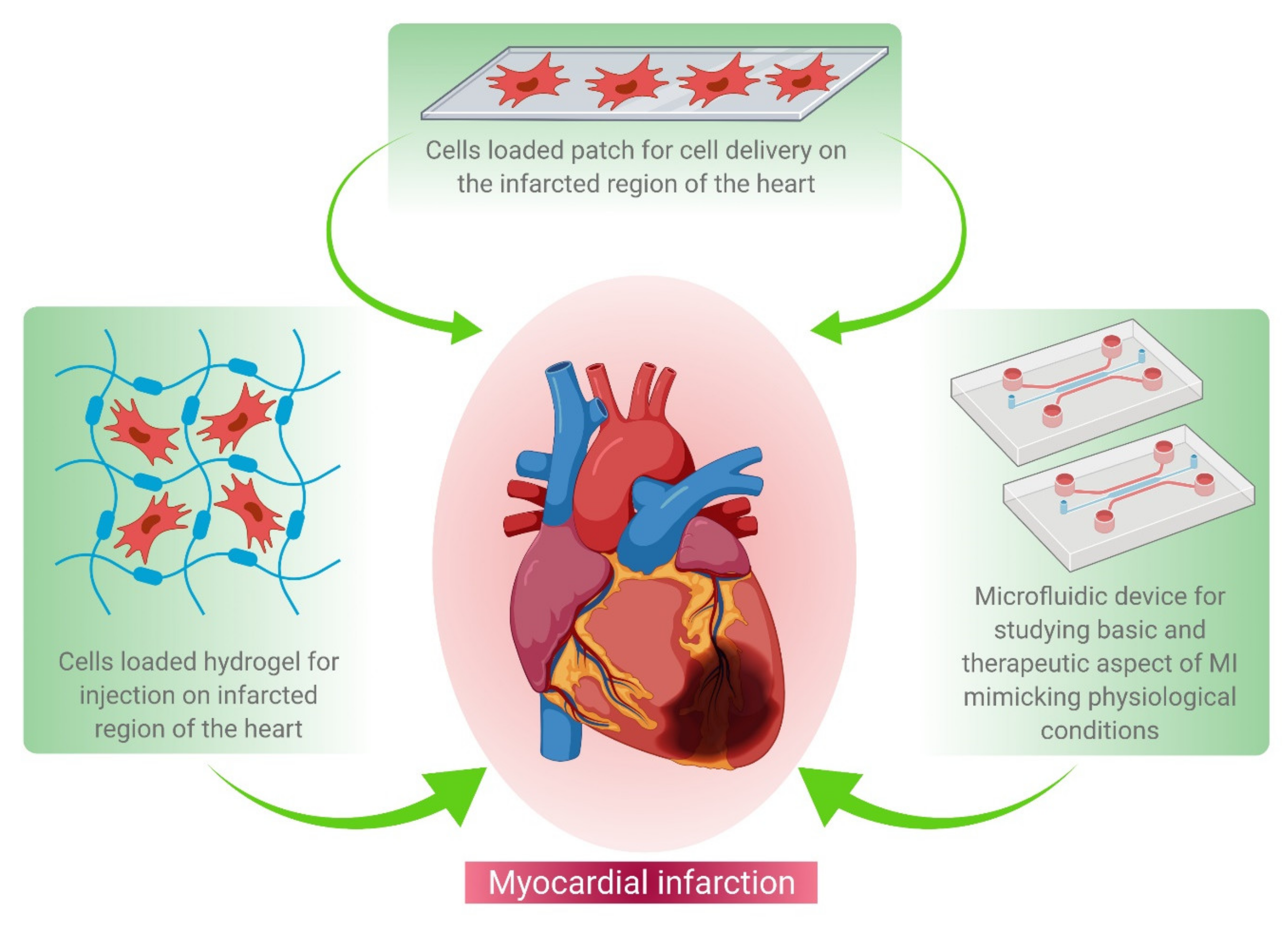
Figure 1. Schematic representation of various tissue engineering approaches for MI treatment. These approaches include hydrogel-based cell delivery (left hand corner), patch-based cell delivery (middle panel), and microfluidics-based drug screening (right corner) during the regenerative therapy of damaged heart tissue.
2. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction
2.1. Regenerative Therapy
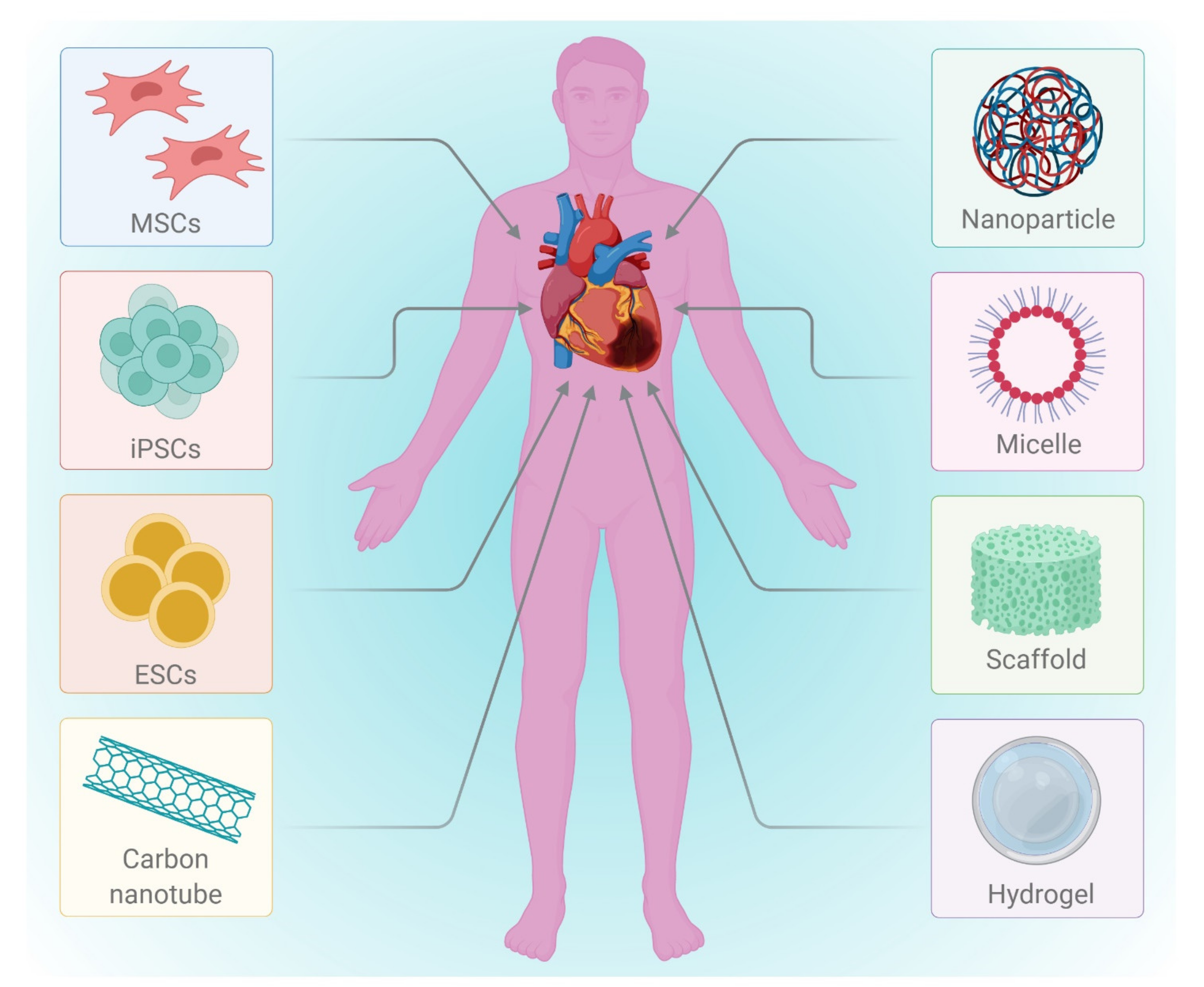
2.2. Cell Based Therapy
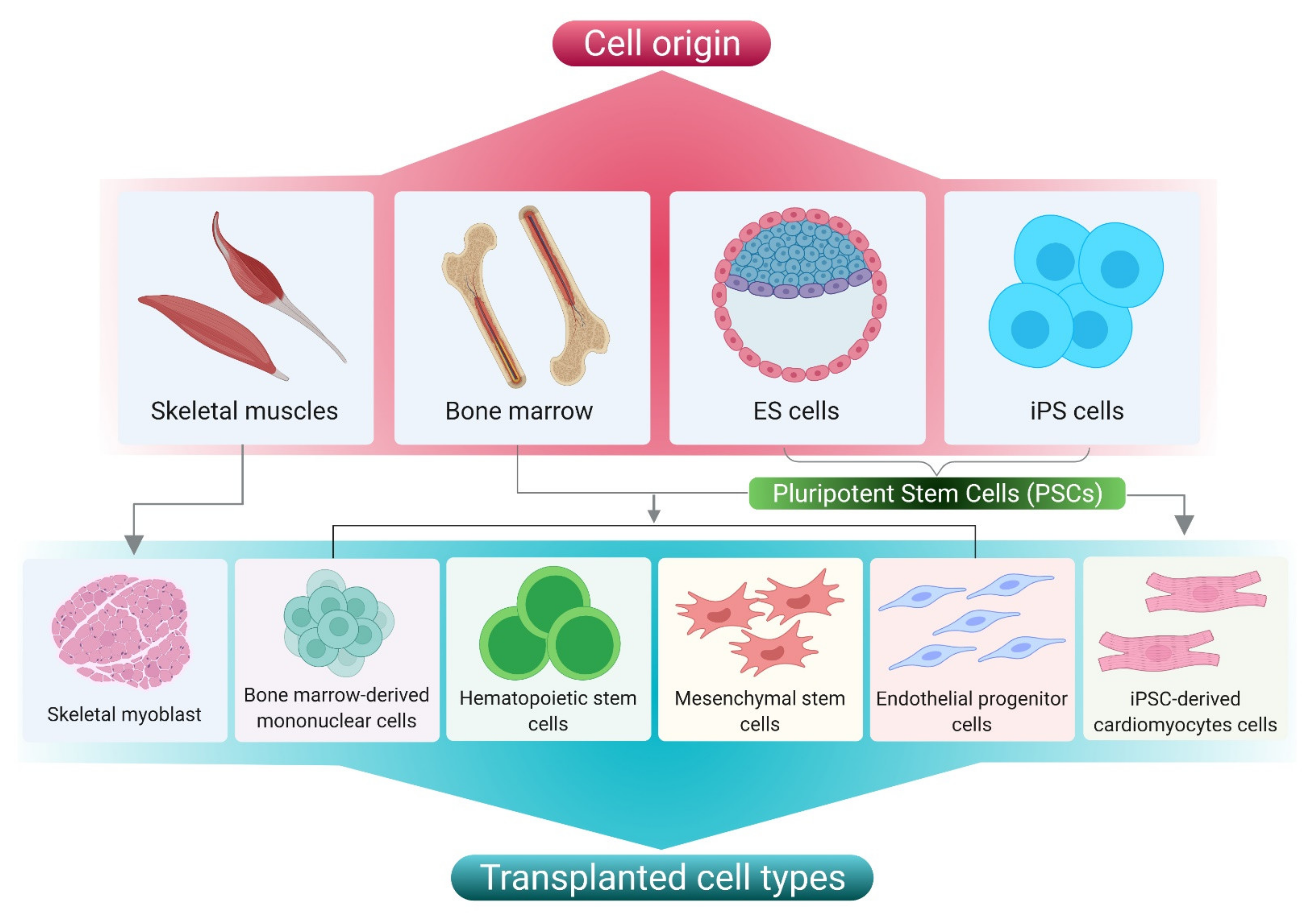
| Initial Cell Type | Target Cell Type | Composition of Delivery Vehicle | Mode of Delivery | Animal Models | Outcomes | Limitations | References |
|---|---|---|---|---|---|---|---|
| iPSCs | CMs | Polyethylene glycol hydrogel | Trans-epicardial | MI in nude rats | Increased infarct thickness and improved muscle content | No donor cell engraftment was observed | [25] |
| Mouse ESCs | CMs | PA-RGDS based gel | Trans-epicardial | Mice | Engraftment and integration of mESC-CMs into host myocardium improved cardiac function |
No information available on cardiac remodelling | [12] |
| iPSCs | CMs | PBS solution | Trans-epicardial | Post-infarcted swine | Enhanced angiogenesis, reduced apoptosis, and blunted cardiac remodelling | No detailed information available on the engraftment of donor cell | [26] |
| MSCs | **** | Self-assembling peptide hydrogels (3-D Matrix, Ltd.) | Surface immobilization by spreading | Lewis rats | Augmented microvascular formation and reduced interstitial fibrosis | No detailed information available on the engraftment of donor cell and CMs differentiation from MSC | [27] |
| MSCs | **** | Si-HPMC | Trans-epicardial | Lewis rats | Short-term recovery of ventricular function and attenuated mid-term remodelling | No detailed information available on the engraftment of donor cell and CMs differentiation from MSC | [28] |
| c-Kit overexpressing CSCs | **** | PBS solution | Intracoronary | Fischer 344 rats | Preserved LV function and structure | Increased cell dose was found to be harmful. Cell tracing or engraftment were not available in detail | [28] |
| CSCs | **** | Matrigel and dimethylpolysiloxane mixture gel | Trans-epicardial | NOD-SCID mice | Improved long-term retention of CSCs, cardiac structure and function | Cell tracing or engraftment were not available | [29] |
2.3. Microfluidics Based MI Research
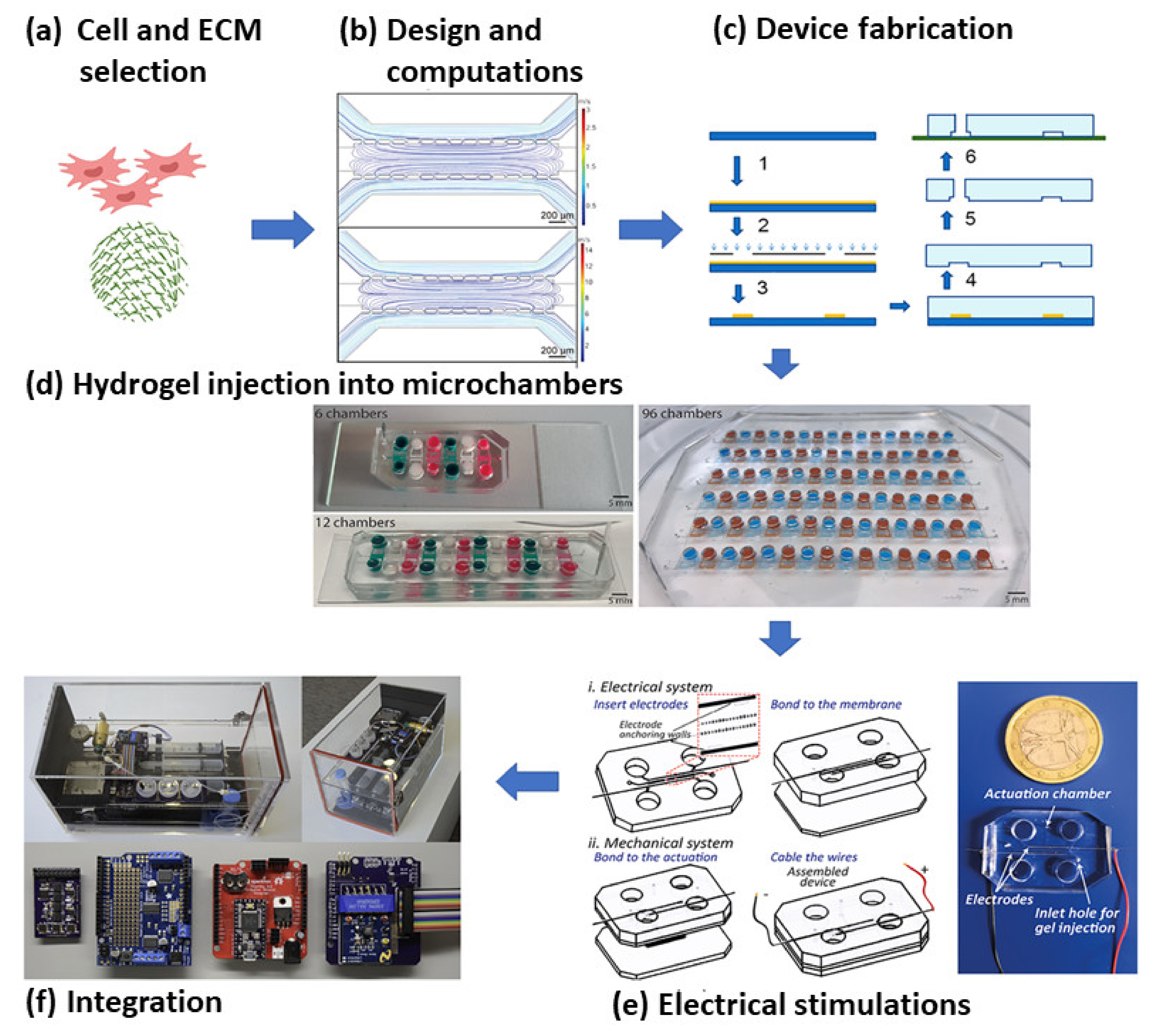
3. Future Directions
This entry is adapted from the peer-reviewed paper 10.3390/cells10102538
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’ 2017 Update. Circulation 2017, 135, e146–e603.
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021. Circulation 2021, 143, 254–743.
- Prabhakaran, D.; Jeemon, P.; Roy, A. Cardiovascular Diseases in India: Current Epidemiology and Future Directions. Circulation 2016, 133, 1605–1620.
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85.
- Pfuntner, A.; Wier, L.M.; Stocks, C. Most Frequent Conditions in U.S. Hospitals, 2011: Statistical Brief#162; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006.
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2015, 5, 1841–1875.
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart transplantation: Challenges facing the field. Cold Spring Harb. Perspect. Med. 2014, 4, a015636.
- Feric, N.T.; Radisic, M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv. Drug Deliv. Rev. 2016, 96, 110–134.
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 2020, 16, 169–187.
- Cahill, T.J.; Choudhury, R.P.; Riley, P.R. Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nat. Rev. Drug Discov. 2017, 16, 699–717.
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623.
- Ban, K.; Park, H.-J.; Kim, S.; Andukuri, A.; Cho, K.-W.; Hwang, J.W.; Cha, H.J.; Kim, S.Y.; Kim, W.-S.; Jun, H.-W.; et al. Cell Therapy with Embryonic Stem Cell-Derived Cardiomyocytes Encapsulated in Injectable Nanomatrix Gel Enhances Cell Engraftment and Promotes Cardiac Repair. ACS Nano 2014, 8, 10815–10825.
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. Npj Regen. Med. 2017, 2, 1–9.
- Chetty, S.S.; Praneetha, S.; Vadivel Murugan, A.; Varthana, K.; Verma, R.S. Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells Labeled with Mn2+ and Gd3+ Co-Doped CuInS2-ZnS Nanocrystals for Multimodality Imaging in a Tumor Mice Model. ACS Appl. Mater. Interfaces 2020, 12, 3415–3429.
- Behfar, A.; Perez-Terzic, C.; Faustino, R.S.; Arrell, D.K.; Hodgson, D.M.; Yamada, S.; Puceat, M.; Niederländer, N.; Alekseev, A.E.; Zingman, L.V.; et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp. Med. 2007, 19, 405–420.
- Kim, J.; Shapiro, L.; Flynn, A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacology 2015, 151, 8–15.
- Ji, L.L.; Long, X.F.; Tian, H.; Liu, Y.F. Effect of transplantation of bone marrow stem cells on myocardial infarction size in a rabbit model. World J. Emerg. Med. 2013, 4, 304–310.
- Roura, S.; Gálvez-Montón, C.; Mirabel, C.; Vives, J.; Bayes-Genis, A. Mesenchymal stem cells for cardiac repair: Are the actors ready for the clinical scenario? Stem Cell Res. Ther. 2017, 8, 1–11.
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 1–8.
- Tang, J.N.; Cores, J.; Huang, K.; Cui, X.L.; Luo, L.; Zhang, J.Y.; Li, T.S.; Qian, L.; Cheng, K. Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem Cells Transl. Med. 2018, 7, 354–359.
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Preclinical Studies of Stem Cell Therapy for Heart Disease. Circ. Res. 2018, 122, 1006–1020.
- Chen, Z.; Chen, L.; Zeng, C.; Wang, W.E. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018, 2018, 7045245.
- Verma, R.S. Recent Advances in Induced Pluripotent Stem Cell (iPSC) based Therapeutics. J. Stem Cell Res. Ther. 2017, 16, 115–130.
- Xu, J.-Y.; Cai, W.-Y.; Tian, M.; Liu, D.; Huang, R.-C. Stem cell transplantation dose in patients with acute myocardial infarction: A meta-analysis. Chronic Dis. Transl. Med. 2016, 2, 92–101.
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422.
- Song, G.; Li, X.; Shen, Y. Qian, L.; Kong, X.; Chen, M.; Cao, K.; Zhang, F. Transplantation of iPSc Restores Cardiac Function by Promoting Angiogenesis and Ameliorating Cardiac Remodeling in a Post-infarcted Swine Model. Cell Biochem. Biophys. 2015, 71, 1463–1473.
- Ichihara, Y.; Kaneko, M.; Yamahara, K.; Koulouroudias, M.; Sato, N.; Uppal, R.; Yamazaki, K.; Saito, S.; Suzuki, K. Self-assembling peptide hydrogel enables instant epicardial coating of the heart with mesenchymal stromal cells for the treatment of heart failure. Biomaterials 2018, 154, 12–23.
- Mathieu, E.; Lamirault, G.; Toquet, C.; Lhommet, P.; Rederstorff, E.; Sourice, S.; Biteau, K.; Hulin, P.; Forest, V.; Weiss, P.; et al. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PLoS ONE 2012, 7, e51991.
- Mayfield, A.E.; Tilokee, E.L.; Latham, N.; McNeill, B.; Lam, B.-K.; Ruel, M.; Suuronen, E.J.; Courtman, D.W.; Stewart, D.J.; Davis, D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 2014, 35, 133–142.
- Inamdar, N.K.; Borenstein, J.T. Microfluidic cell culture models for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 681–689.
- Ni, M.; Tong, W.H.; Choudhury, D.; Rahim, N.A.A.; Iliescu, C.; Yu, H. Cell culture on MEMS platforms: A review. Int. J. Mol. Sci. 2009, 10, 5411–5441.
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373.
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morphology, and Alignment. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 536–546.
- Qiao, Y.; Dong, Q.; Li, B.; Obaid, S.; Miccile, C.; Yin, R.T.; Talapatra, T.; Lin, Z.; Li, S.; Li, Z.; et al. Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Prog. Biophys. Mol. Biol. 2019, 144, 139–150.
- Visone, R.; Talò, G.; Occhetta, P.; Cruz-moreira, D.; Lopa, S.; Pappalardo, O.A.; Redaelli, A.; Moretti, M.; Rasponi, M. A microscale biomimetic platform for generation and electro-mechanical stimulation of 3D cardiac microtissues. APL Bioeng. 2018, 2, 046102.
- Visone, R.; Ugolini, G.S.; Vinarsky, V.; Penati, M.; Redaelli, A.; Forte, G.; Rasponi, M. A Simple Vacuum-Based Microfluidic Technique to Establish High-Throughput Organs-On-Chip and 3D Cell Cultures at the Microscale. Adv. Mater. Technol. 2019, 4, 1–8.
