Enterococci derived from an ancestor that was a commensal of aquatic life forms, when animals first became terrestrial. To cope with the stresses of the new terrestrial habitat, enterococci evolved to be tough bugs, resistant to a wide range of environmental and host factors. That made them extremely successful not only in adapting to the new way of life of their hosts, but also in colonizing other non-animal, and even inanimate, environments - such as feeds and foods. The plasticity of the enterococcal genome, together with their notable ability to trade virulence and antibiotic resistance, have enabled them to also become notable opportunistic, multi-resistant pathogens and act as reservoirs of pathogenicity and resistance determinants.
- Enterococcus
- enterococci
- commensal
- opportunistic pathogen
- antibiotic resistance
1. Introduction
Traditionally, enterococci were regarded as a harmless commensal bacterium, and were even believed to have positive effects on a number of gastrointestinal and systemic conditions. However, when the commensal relationship with the host is disrupted, enterococci can cause invasive infections [1]. Enterococci are Gram-positive catalase-negative, non-spore-forming, facultative anaerobic lactic acid bacteria, and normal inhabitants of the gut flora of humans, many different mammals, birds, fish, reptiles, amphibians and insects, as well as nematodes [2][3]. Until 1984, the enterococci were considered as part of the genus Streptococcus, but they have constituted a unique taxonomic entity since the mid-1980s [4][5]. Today, over 50 different species of enterococci have been described, of which Enterococcus faecium and E. faecalis are the most common in the human gastrointestinal tract, whereas, among farm animals E. faecium together with E. cecorum, E. faecalis and, to some extent, E. hirae predominate, while E. mundtii and E. casseliflavus are commonly found in plant sources [6][7]. Moreover, ecology and epidemiological studies have reported E. faecalis and E. faecium as frequently isolated from food products (cheese, fish, sausages, minced beef, and pork) and the environment (sewage, soil, and water) [8]. Due to their preferred intestinal habitat, their wide occurrence, robustness, and ease of cultivation, enterococci are used as indicators of fecal contamination and are part of the hygiene standards for water and food products. Additionally, they are also suitable as important key indicator bacteria for veterinary and human antimicrobial resistance surveillance systems [5].
Because they produce bacteriocins, enterococcal isolates have a long-standing tradition as starter cultures or as supplements in food fermentation and food preservation [9]. Bacteriocin ST15 from E. mundtii has been shown to be effective against a range of Gram-positive and Gram-negative bacteria including Acinetobacter, Bacillus, Clostridium, Klebsiella, Lactobacillus, and Pseudomonas, whereas enterocins A, B, I, L and P, are active against Listeria species, Clostridium species, and Staphylococcus aureus [10]. Another beneficial aspect is the use of E. faecalis as a probiotic, promoting a positive gut environment. Additionally, enterococci have been shown to strengthen the immune system, reduce inflammation, and may even be indirectly involved in reducing the incidence of colon cancer [11].
Enterococci are very hardy organisms; they evolved to endure various adverse conditions and survive for several months in the environment [6]. They are able to survive in a range of stressful and hostile environments, including extreme conditions of pH and temperatures (between 10 °C and 45 °C), plus high NaCl concentration [8]. These attributes make enterococci ideally suited for fermentation applications but, ironically, these same attributes make them difficult to eliminate and control once they become established in a hospital environment. Therefore, enterococci are now firmly established as one of the major nosocomial pathogens and are increasingly becoming more resistant to antimicrobial agents. Presently, almost all nosocomial enterococcal infections are caused by either E. faecalis or E. faecium[6]. Of these, E. faecalis is the most virulent species, but E. faecium is of increasing importance as, in general, it frequently is more resistant to antimicrobials [4]. Commonly, these organisms are involved in hospital-acquired infections such as catheter-associated urinary tract infections, endocarditis, bacteremia, neonatal sepsis, surgical and burn wound infections, and more rarely meningitis [6].
Enterococci are typically harmless in healthy individuals. They become opportunistic pathogens mainly by causing infections in patients who are in Intensive Care Units, who suffer from a severe underlying disease, or who are immunocompromised. Therefore, the severity of illness and immune suppression can be directly associated with prolonged hospital and/or indiscriminate antibiotics use, and these are major risk factors for nosocomial acquisition of drug-resistant enterococci [12]. Antibiotic treatment may create new niches and nutrient resources, wherefore the existing commensal gut microbiota can be eliminated and subsequently replaced by opportunistic pathogens [13]. Patients in hospitals are typically treated with broad-spectrum antibiotics (penicillins and cephalosporins), which dramatically increases hospital-associated E. faecium colonization in their small intestine, cecum, and colon, outcompeting the normal Gram-negative gut microbiota [14][15]. In addition, some antibiotic therapies may lower the levels of C-type lectin RegIII produced by the host, allowing E. faecium to overgrow in the intestinal tract [15]. Furthermore, the intrinsic resistance of enterococci to several commonly used antibiotics and, perhaps more importantly, their malleable genomes plus their capacity to acquire and disseminate determinants of antibiotic resistance, are major factors that may have contributed to their adaptation to harsh environments [16]. Both microbial and host factors can contribute to the conversion of a second-rate pathogen into a first-rate clinical problem, as is seen nowadays.
2. Persistence of VRE Strains in Portuguese Food Animals, an Example
Vancomycin is still a frequently used antibiotic to treat infections caused by multiresistant enterococci, despite the present expansion of VRE strains representing a tremendous challenge to human infection control. In Europe, as previously explained, it has been suggested that the massive use of avoparcin in animal husbandry was associated with the high prevalence rates of VRE strains detected in food-producing animals and their subsequent expansion into the community. Once the use of avoparcin was discontinued, the prevalence of VRE among farm animals decreased.
Nevertheless, VRE are still present among farm animals. Figure 1 compares the prevalence of VRE reported in different settings in Portugal (food-producing animals, environmental, wastewater treatment plants, wild animals, and pets). The displayed data show that VRE strains are still broadly distributed in Portugal, being isolated not only from healthy food-producing animals, wild animals, and pets, but also from the environment. VRE are found in wastewater treatment plants and in pig breeding facilities. The environmental prevalence of VRE is troublesome, as the release of effluents into watercourses and the use of sludge in agriculture might actively contribute to the dissemination of VRE strains, resistant bacteria and resistance genes throughout the environment [17].
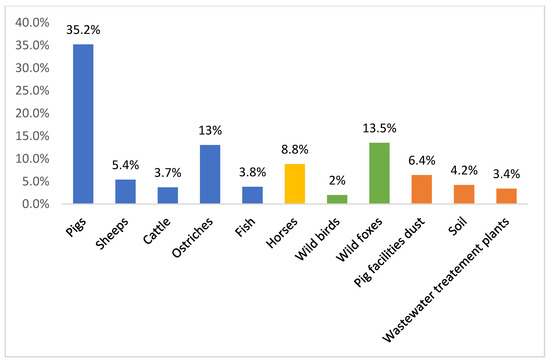
Figure 1. Percentages of vancomycin-resistant enterococci reported in Portugal in different settings: food-producing animals, environmental, wastewater treatment plants, wild animals, horses, and pets [18][19][20][21][22][23][24][25][26].
Furthermore, the persistence of VRE in food-producing animals and related environments (years after avoparcin withdrawal) indicates that coselection with other antimicrobial agents increased fitness of strains and the presence of specific mobile genetic elements cannot be ruled out. It is known that the ermB gene, encoding for macrolide resistance, can be carried by the same conjugative plasmid harboring vanA gene [27]. Moreover, a putative linkage of the glycopeptide, macrolide, and tetracycline-resistant genes has been implicated in the occurrence of VRE in the feces of food-producing animals [16]. In fact, tetracycline and macrolides are widely prescribed in pig husbandry to control respiratory and enteric disease and the use of one of these antimicrobials may favor the spread of resistance against antimicrobial from different groups. In a recent study, all VRE strains with acquired mechanisms of resistance (VREar) from pigs showed coresistance to tetracycline and erythromycin, which supports the hypothesis that this linkage could be a causative factor for the ongoing persistence of VRE [18]. It is important to notice that the mobile element Tn916/Tn1545-like transposon was detected in the majority of our VREar strains. This is consistent with the results from other Portuguese settings, where these mobile genetic elements were also frequently associated with acquired vancomycin resistance [21][25]. Moreover, it was reported, in Portugal, that human and swine share vancomycin-resistant E. faecium strains harboring Tn1546 on indistinguishable plasmids [28]. In addition, the rapid and extensive spread of VRE in Portuguese hospitals seems to be associated with the dissemination of the vanA gene on Tn1546-type transposons [28]. Nonetheless, VRE persistence, being continuously reported among animals and the environment, shows that this data should not be overlooked and must be continuously monitored.
In addition to food-producing animals, VRE and their resistance genes have been reported and detected in foods at retail (meat, vegetables, cheese, and milk) [29][30][31][32]. This is a cause for enormous concern since it favors the dissemination of antimicrobial resistance organisms, consequently reducing therapeutic options [33]. In Portugal, only a few studies have reported the presence of VRE in food such as cheese, poultry carcasses, and processed meat [29][34][35][36][37][38][39].
3. Findings
At present, our knowledge regarding the occurrence of antimicrobial resistance in food-producing animals, the quantitative impact by the use of different antimicrobial agents on the selection of resistance, and the most appropriate treatment protocols to limit the development of resistance still have some limitations. Prevalence of antimicrobial resistance in studies on food-producing animals contributes towards establishing a knowledge base regarding the emergence of resistant bacteria. Accordingly, programs monitoring the occurrence and development of resistance and consumption of antimicrobial agents are strongly desirable, as is research into the most appropriate ways to use antimicrobial. In turn, this could aid in the implementation of guidelines and regulations on the usage of antimicrobial agents in livestock production systems. Such guidelines for the prudent use of antimicrobial agents may help to slow down the selection for resistance, should be based on knowledge regarding the normal susceptibility patterns of the causative agents, and take into account the potential problems for human health.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8081118
References
- Wendy Escobedo-Hinojosa; Liliana Pardo-López; Analysis of bacterial metagenomes from the Southwestern Gulf of Mexico for pathogens detection. Pathogens and Disease 2017, 75, 103430, 10.1093/femspd/ftx058.
- Wendy Escobedo-Hinojosa; Liliana Pardo-López; Analysis of bacterial metagenomes from the Southwestern Gulf of Mexico for pathogens detection. Pathogens and Disease 2017, 75, ftx058, 10.1093/femspd/ftx058.
- Carolina Baldisserotto Comerlato; Ana Carolina Ritter; Kendi Nishino Miyamoto; Adriano Brandelli; Proteomic study of Enterococcus durans LAB18S growing on prebiotic oligosaccharides. Food Microbiology 2020, 89, 103430, 10.1016/j.fm.2020.103430.
- O. Nilsson; Vancomycin resistant enterococci in farm animals – occurrence and importance. Infection Ecology & Epidemiology 2012, 2, 606, 10.3402/iee.v2i0.16959.
- Guido Werner; Teresa M. Coque; Charles M.A.P. Franz; Elisabeth Grohmann; Kristin Hegstad; L.B. Jensen; Willem Van Schaik; Keith Weaver; Antibiotic resistant enterococci—Tales of a drug resistance gene trafficker. International Journal of Medical Microbiology 2013, 303, 360-379, 10.1016/j.ijmm.2013.03.001.
- Krista Dubin; Eric G. Pamer; Enterococci and Their Interactions with the Intestinal Microbiome. Mobile DNA III 2018, 5, 309-330, 10.1128/microbiolspec.bad-0014-2016.
- Rahat Zaheer; Shaun R. Cook; Ruth Barbieri; Noriko Goji; Andrew Cameron; Aaron Petkau; Rodrigo Ortega Polo; Lisa Tymensen; Courtney Stamm; Jiming Song; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Scientific Reports 2020, 10, 1-16, 10.1038/s41598-020-61002-5.
- Carmen Torres; Carla Alonso; Laura Ruiz-Ripa; Ricardo Leon-Sampedro; Rosa Del Campo; Teresa M. Coque; Antimicrobial Resistance in Enterococcus spp. of animal origin. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals 2018, 6, 185-227, 10.1128/microbiolspec.arba-0032-2018.
- Xiaoxiao Qiao; Renpeng Du; Yu Wang; Ye Han; Zhijiang Zhou; Isolation, Characterisation and Fermentation Optimisation of Bacteriocin-Producing Enterococcus faecium. Waste and Biomass Valorization 2019, 11, 1-9, 10.1007/s12649-019-00634-9.
- K. Fisher; Carol Phillips; The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749-1757, 10.1099/mic.0.026385-0.
- Carolina Vieira De Almeida; Antonio Taddei; Amedeo Amedei; The controversial role of Enterococcus faecalis in colorectal cancer. Therapeutic Advances in Gastroenterology 2018, 11, 1756284818783606, 10.1177/1756284818783606.
- Talat A. El-Kersh; Mohammed A. Marie; Yazeed A. Al-Sheikh; Mohamed H.M. Al-Agamy; Ahmad A. Al Bloushy; Prevalence and risk factors of early fecal carriage of Enterococcus faecalis and Staphylococcus spp and their antimicrobial resistant patterns among healthy neonates born in a hospital setting in central Saudi Arabia. Saudi Medical Journal 2016, 37, 280-287, 10.15537/smj.2016.3.13871.
- Liu, L.; Wang, Q.; Wu, X.; Qi, H.; Das, R.; Lin, H.; Shi, J.; Wang, S.; Yang, J.; Xue, Y.; et al. Vancomycin exposure caused opportunistic pathogens bloom in intestinal microbiome by simulator of the human intestinal microbial ecosystem (SHIME). Environmental Pollution 2020, 265, 114399, j.envpol.2020.114399.
- Cesar A. Arias; Barbara E. Murray; The rise of the Enterococcus: beyond vancomycin resistance. Nature Reviews Genetics 2012, 10, 266-278, 10.1038/nrmicro2761.
- Antoni P.A. Hendrickx; Willem Van Schaik; Rob J.L. Willems; The cell wall architecture ofEnterococcus faecium: from resistance to pathogenesis. Future Microbiology 2013, 8, 993-1010, 10.2217/fmb.13.66.
- Mónica García-Solache; Louis B. Rice; The Enterococcus: a Model of Adaptability to Its Environment. Clinical Microbiology Reviews 2019, 32, e00058-18, 10.1128/cmr.00058-18.
- Mutshiene Deogratias Ekwanzala; John Barr Dewar; Ilunga Kamika; Maggy Ndombo Benteke Momba; Comparative genomics of vancomycin-resistant Enterococcus spp. revealed common resistome determinants from hospital wastewater to aquatic environments. Science of The Total Environment 2020, 719, 137275, 10.1016/j.scitotenv.2020.137275.
- Sónia Ramos; Gilberto Igrejas; J. Rodrigues; José L. Capelo-Martínez; Patrícia Poeta; Genetic characterisation of antibiotic resistance and virulence factors in vanA-containing enterococci from cattle, sheep and pigs subsequent to the discontinuation of the use of avoparcin. The Veterinary Journal 2012, 193, 301-303, 10.1016/j.tvjl.2011.12.007.
- Carlos Araújo; Carmen Torres; Alexandre Gonçalves; Catarina Carneiro; María López; Hajer Radhouani; Miguel Ângelo Pardal; Gilberto Igrejas; Patrícia Poeta; Genetic Detection and Multilocus Sequence Typing ofvanA-ContainingEnterococcusStrains from Mullets Fish (Liza ramada). Microbial Drug Resistance 2011, 17, 357-361, 10.1089/mdr.2010.0171.
- Carlos Araújo; Carmen Torres; Nuno Silva; Catarina Carneiro; Alexandre Gonçalves; Hajer Radhouani; Susana Correia; Paulo Martins Da Costa; Rui Pacheco; Myriam Zarazaga; et al. Vancomycin-resistant enterococci from Portuguese wastewater treatment plants. Journal of Basic Microbiology 2010, 50, 605-609, 10.1002/jobm.201000102.
- Alexandre Gonçalves; Patrícia Poeta; Nuno Silva; Carlos Araújo; María López; Elena Ruiz; Inna Uliyakina; João Direitinho; Gilberto Igrejas; Carmen Torres; et al. Characterization of Vancomycin-Resistant Enterococci Isolated from Fecal Samples of Ostriches by Molecular Methods. Foodborne Pathogens and Disease 2010, 7, 1133-1136, 10.1089/fpd.2010.0548.
- Ines Moura; Hajer Radhouani; Carmen Torres; Patrícia Poeta; G. Igrejas; Detection and genetic characterisation of vanA-containing Enterococcus strains in healthy Lusitano horses. Equine Veterinary Journal 2010, 42, 181-183, 10.2746/042516409x480386.
- Hajer Radhouani; Gilberto Igrejas; Carlos Carvalho; Luís Pinto; Alexandre Gonçalves; María López; Roberto Sargo; Luís Cardoso; António Martinho; Vítor Rego; et al. Clonal Lineages, Antibiotic Resistance and Virulence Factors in Vancomycin-Resistant Enterococci Isolated from Fecal Samples of Red Foxes (Vulpes Vulpes). Journal of Wildlife Diseases 2011, 47, 769-773, 10.7589/0090-3558-47.3.769.
- Teresa M. Braga; Constança Pomba; Maria De Fátima Silva Lopes; High-level vancomycin resistant Enterococcus faecium related to humans and pigs found in dust from pig breeding facilities. Veterinary Microbiology 2012, 161, 344-349, 10.1016/j.vetmic.2012.07.034.
- Vanessa Silva; Gilberto Igrejas; Isabel Carvalho; Fernando Peixoto; Luís Cardoso; Jose E. Pereira; Rosa Del Campo; Patrícia Poeta; Genetic Characterization of vanA-Enterococcus faecium Isolates from Wild Red-Legged Partridges in Portugal. Microbial Drug Resistance 2017, 24, 89-94, 10.1089/mdr.2017.0040.
- Vanessa Silva; Fernando Peixoto; Gilberto Igrejas; Carolina Parelho; Patricia V. Garcia; Isabel Carvalho; Margarida Sousa; José Pereira; Armindo Dos Santos Rodrigues; Patrícia Poeta; et al. First Report on vanA-Enterococcus faecalis Recovered from Soils Subjected to Long-Term Livestock Agricultural Practices in Azores Archipelago. International Journal of Environmental Research 2018, 12, 39-44, 10.1007/s41742-018-0068-0.
- Frank M. Aarestrup; Characterization of Glycopeptide-Resistant Enterococcus faecium (GRE) from Broilers and Pigs in Denmark: Genetic Evidence that Persistence of GRE in Pig Herds Is Associated with Coselection by Resistance to Macrolides. Journal of Clinical Microbiology 1999, 38, 2774-2777, 10.1128/jcm.38.7.2774-2777.2000.
- Ana R. Freitas; Teresa M. Coque; Carla Novais; Anette M. Hammerum; Camilla H. Lester; Marcus J. Zervos; Susan Donabedian; L.B. Jensen; Maria Victoria Francia; Fernando Baquero; et al. Human and Swine Hosts Share Vancomycin-Resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 Clonal Clusters Harboring Tn1546 on Indistinguishable Plasmids. Journal of Clinical Microbiology 2011, 49, 925-931, 10.1128/jcm.01750-10.
- Carolina Sabença; Telma De Sousa; Soraia Oliveira; Didier Viala; Laetitia Théron; Christophe Chambon; Michel Hébraud; Racha Beyrouthy; Richard Bonnet; Manuela Caniça; et al. Next-Generation Sequencing and MALDI Mass Spectrometry in the Study of Multiresistant Processed Meat Vancomycin-Resistant Enterococci (VRE). Biology 2020, 9, 89, 10.3390/biology9050089.
- Ramin Mazaheri Nezhad Fard; Payam Haghighi Khoshkhoo; Maryam Abbaspour; Zahra Rajabi; Study of VanA, B, C, D, E Genes in Vancomycin Resistant Enterococci Isolated from Retailed Dried Vegetables in Tehran, Iran. International Journal of Enteric Pathogens 2019, 7, 9-14, 10.15171/ijep.2019.03.
- Ali Jahansepas; Yaeghob Sharifi; Mohammad Aghazadeh; Mohammad Ahangarzadeh Rezaee; Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: antimicrobial susceptibility and virulence traits. Archives of Microbiology 2019, 202, 765-772, 10.1007/s00203-019-01792-z.
- Ali Hassan Ahmed Al-Shammary; Run-off Patterns of Vancomycin Resistant Enterococci (VRE clones) in Cows Raw Milk and Imported Milk Powders at Baghdad Markets. Iraqi Journal of Veterinary Medicine 2019, 43, 61-66, 10.30539/iraqijvm.v43i2.532.
- Vanessa Pereira Perez Alonso; Murilo M Queiroz; Miriã L Gualberto; M.S. Nascimento; Klebsiella pneumonia carbapenemase (KPC), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus spp. (VRE) in the food production chain and biofilm formation on abiotic surfaces. Current Opinion in Food Science 2019, 26, 79-86, 10.1016/j.cofs.2019.04.002.
- Carla Novais; T. M. Coque; M. J. Costa; J. C. Sousa; F. Baquero; Luísa Peixe; High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. Journal of Antimicrobial Chemotherapy 2005, 56, 1139-1143, 10.1093/jac/dki360.
- Ana R. Freitas; Carla Novais; Rosa Correia; Márcia Monteiro; Teresa M. Coque; Luísa Peixe; Non-susceptibility to tigecycline in enterococci from hospitalised patients, food products and community sources. International Journal of Antimicrobial Agents 2011, 38, 174-176, 10.1016/j.ijantimicag.2011.04.014.
- Joana Barbosa; Vânia Borges Ferreira; Paula Teixeira; Antibiotic susceptibility of enterococci isolated from traditional fermented meat products. Food Microbiology 2009, 26, 527-532, 10.1016/j.fm.2009.03.005.
- Ribeiro, T.; Oliveira, M.; Fraqueza, M.J.; Lauková, A.; Elias, M.; Tenreiro, R.; Barreto, A.S.; SemedoLemsaddek, R.; Antibiotic resistance and virulence factors among enterococci isolated from chouriço, a raditional Portuguese dry fermented sausage. Journal of Food Protection 2011, 74, 465–469, 10.4315/0362-028X.JFP- 10-309.
- M.F.P. Domingos-Lopes; Catherine Stanton; P.R. Ross; Maria De Lurdes Enes Dapkevicius; C.C. Silva; R. Paul Ross; Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiology 2017, 63, 178-190, 10.1016/j.fm.2016.11.014.
- Câmara, S.P.A.; Dapkevicius, A.; Silva, C.C.G.; Malcata, F.X.; Dapkevicius, M.L.N.E.; Artisanal Pico cheese as reservoir of Enterococcus species possessing virulence and antibiotic resistance properties: implications for food safety. Food Biotechnology 2020, 34, 25–41, 10.1080/08905436.2019.1710844.pira.
