Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dentistry, Oral Surgery & Medicine
Dry mouth is common among older people, affecting between 20% and 30% of those over 65. There are two aspects to the condition. Salivary gland hypofunction (SGH) is the state of having low salivary flow, while xerostomia refers to the subjective sensation of dry mouth.
- xerostomia
- dry mouth
- polypharmacy
- residential aged care
1. Introduction
Dry mouth is particularly common in residential aged care, reported in about one in three residents in a recent New Zealand (NZ) national survey [1].
2. Occurrence and Impact of Dry Mouth
Dry mouth is common among older people, affecting between 20% and 30% of those over 65 [2][3]. There are two aspects to the condition. Salivary gland hypofunction (SGH) is the state of having low salivary flow, while xerostomia refers to the subjective sensation of dry mouth. Thus, the former is a sign, and the latter a symptom (or set of symptoms). The extent to which they coincide is controversial, but the epidemiological evidence suggests that they are far from concordant [4].
By no means a trivial condition, dry mouth has a considerable impact on sufferers (Table 1). Not only is it one of the major contributors to impaired oral-health-related quality of life (OHRQoL) among adults of any age [5][6][7][8][9][10][11], but sufferers of dry mouth have difficulty eating and swallowing, halitosis, poor sleep, and considerably higher tooth decay rates [12], along with difficulty wearing dental prostheses (partial or complete dentures).
Table 1. Overview of dry mouth’s impacts on sufferers.
| Physical and Functional Impacts | Psychosocial Impacts |
|---|---|
| Difficulty eating/swallowing | Symptoms of dry mouth |
| More tooth decay | Compromised quality of life |
| Problems with dentures | Halitosis |
| Infections—salivary glands, mucosa | Poor sleep |
| Compromised taste sensation | Distress |
3. Causes of Dry Mouth
Medications are the most important risk factor for chronic dry mouth [2]. Although a small proportion of people suffer from the condition as a result of autoimmune conditions such as Sjögren’s syndrome, or as a side-effect of radiotherapy for head/neck cancer, more than 95% of the population burden of dry mouth arises as a result of medication use.
Drugs that are putative causes of dry mouth are referred to as “xerogenic”. Lists of xerogenic medications have been published [13][14][15] but are of limited utility because they are too inclusive and largely based on case reports. Medication classes for which there is sound evidence of xerogenicity include antidepressants, anticholinergics, opioids and bronchodilators (Table 2).
Table 2. The main medication classes known to be associated with dry mouth (adapted from Villa et al. [4]).
| Medication Type | Mechanism of Action |
|---|---|
| Gastrointestinal agents e.g., Hyoscine, hyoscyamine, belladonna alkaloids, atropine |
Block muscarinic receptors |
| Antiemetics e.g., prochlorperazine, |
Block dopamine D2, serotonin types 2–4, histamine type 1 and acetylcholine receptors |
| Appetite suppressants/stimulants e.g., Phentermine, sibutramine |
Inhibit CNS uptake of norepinephrine, serotonin and dopamine |
| Cardiovascular agents e.g., Atenolol, metoprolol, prazosin, clonidine |
Block α1- and β2-adrenergic receptors |
| Urological e.g., Oxybutynin, propantheline, darifenacin, solifenacin, tolterodine, mirabegron |
Block muscarinic receptors and α1-adrenergic receptors |
| Muscle relaxants Cyclobenzaprine, orphenadrine |
Act as α1-adrenergic receptor agonists, and H2 histamine blockers |
| Analgesics e.g., Opioids, tramadol, gabapentin, pregabalin. |
Block noradrenaline reuptake in the CNS and so inhibit the salivary reflex arc |
| Anticonvulsants e.g., Carbamazepine |
Act centrally to reduce neurotransmitter release |
| Sedatives—benzodiazepines & Z-drugs e.g., Zolpidem, zopiclone |
Enhance GABA effect in CNS, reduce the salivary secretory reflex, and block muscarinic, α1- and β2-adrenergic receptors |
| Antipsychotics e.g., Olanzapine, clozapine, amisulpiride |
Block neurotransmitter uptake (various) |
| Antidepressants e.g., Tricyclics (e.g., amitriptyline), SSRIs and SNRIs |
Anticholinergic; increase serototinn and noradrenaline at the synaptic cleft. |
| Bronchodilators e.g., Ipratropium, tiotropium, salbutamol, salmeterol, eformoterol, umeclidinium |
2 types: β agonists and antimuscarininc, Block muscarinic receptors M1 and M3, |
| Antihistamines- sedating only e.g., Diphenhydramine, doxylamine, chlorpheniramine, promethazine |
Central inhibitory action on histamine type 1 and muscarinic receptors |
| CNS Stimulants e.g., Caffeine, pseudoephedrine, amphetamines |
α1 and α2 agonists. |
Other factors being equal, people taking large numbers of different medications have higher rates of dry mouth. That is, the greater the number and dosage of drugs being taken, the greater the severity of dry mouth (and other side-effects). In the NZ national survey of residential aged care, xerostomia was more common among those taking 5–9 medications and more so in those on 10+ medications, as well as in those taking antidepressants or bronchodilators [1].
4. Treating Dry Mouth and Reducing Unnecessary Medication Use
Dry mouth is difficult to treat. Broadly speaking, the therapeutic options are palliation (treating the symptoms), stimulation (increasing salivary gland output) or regeneration (growing new secretory tissue). The latter remains a theoretical possibility at this stage, while stimulation has had mixed outcomes. Palliative approaches can be inconsistent and unpredictable, as shown in Cochrane reviews [16][17][18], which have examined the evidence for the different therapeutic approaches. There is strong evidence for the efficacy of stimulation using systemic pilocarpine (in individually titrated doses of, typically, 2–5 mg) in treating dry mouth arising from Sjögren’s syndrome or from therapeutic radiation for head/neck cancer treatment, but those states comprise only a very small proportion of the population burden arising from dry mouth [19].
Given that the bulk of the population-attributable risk for dry mouth arises from medications (and polypharmacy in particular)—and that the evidence for stimulatory approaches to treating medication-related dry mouth is not strong—there is a need to examine alternative ways to prevent or ameliorate it by reducing the occurrence of polypharmacy in residential aged care.
Accordingly, interventions aiming to reduce the occurrence of dry mouth through medication review and deprescribing (and thereby reducing polypharmacy) would be a key strategy with important benefits, not only for older people and residential aged care facilities, but also for the wider health system. What is medication review? It involves a systematic, critical assessment of a patient’s medicines, which aims to arrive at an agreement with the patient on treatment, optimising the impact of medicines, and minimising medication-related problems and waste. An essential part of that process is medication reconciliation, the assembling of an accurate and complete inventory of all medications taken, regardless of source [20]. Medication review itself can have four levels [21] (Figure 1). (a) Prescription review considers the technical features of the prescription itself. (b) Adherence support review is undertaken with the patient present and focuses on medication-taking, particularly knowledge and adherence. (c) Clinical review involves the clinical notes and the patient, considering their use of medications with respect to their clinical condition. (d) Clinical review with prescribing is an extension of the former but including the authority to prescribe. Levels (c) and (d) closely involve the patient’s physician; overall, the focus should be on assessing the medication’s risks and benefits, and initiating deprescribing for those where the former outweigh the latter.
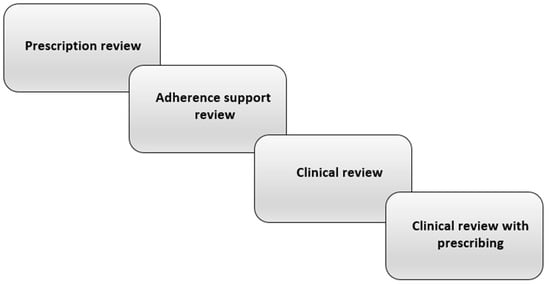
Figure 1. The levels of pharmacist-led medication review [21].
A useful distinction in undertaking medication entry can be made between drugs being taken for control of disease and/or symptoms and those taken for preventive reasons, despite a degree of overlap [22]. In a recent investigation of medications causing dry mouth in a national survey of residential aged care in New Zealand, the types most strongly associated with dry mouth were antidepressants, corticosteroids, anticholinergics and bronchodilators [1]. These are prescribed largely for symptom control rather than disease control in older people, and so any deprescribing moves would need to be informed by considering the benefit:harm ratio and the likelihood of withdrawal reactions or disease rebound on cessation, along with patient and physician preferences [23].
Pharmacists can play an important role in the above process, particularly through working together with dentists, since medical practitioners may be more likely to act upon recommendations made by two practitioners from different fields.
This entry is adapted from the peer-reviewed paper 10.3390/pharmacy9040162
References
- Thomson, W.M.; Ferguson, C.A.; Janssens, B.E.; Kerse, N.M.; Ting, G.S.; Smith, M.B. Xerostomia and polypharmacy among dependent older New Zealanders: A national survey. Age Aging 2021, 50, 248–251.
- Agostini, B.A.; Cericato, G.O.; Silveira, E.R.; Nascimento, G.G.; Costa, F.S.; Thomson, W.M.; Demarco, F.F. How common is dry mouth? Systematic review and meta-regression analysis of prevalence estimates. Braz. Dent. J. 2018, 29, 606–618.
- Jamieson, L.M.; Thomson, W.M. Xerostomia: Its prevalence and associations in the adult Australian population. Aust. Dent. J. 2020, 65, S67–S70.
- Villa, A.; Wolff, A.; Narayana, N.; Dawes, C.; Aframian, D.; Lynge Pedersen, A.M.; Vissink, A.; Aliko, A.; Sia, Y.W.; Joshi, R.K.; et al. World Workshop on Oral Medicine VI: A systematic review of medication-induced salivary gland dysfunction: Prevalence, diagnosis, and treatment. Clin. Oral Investig. 2016, 22, 365–382.
- Locker, D. Dental status, xerostomia and the oral health-related quality of life of an elderly institutionalized population. Spec. Care Dent. 2003, 23, 86–93.
- Gerdin, E.W.; Einarson, S.; Jonsson, M.; Aronsson, K.; Johansson, I. Impact of dry mouth conditions on oral health-related quality of life in older people. Gerodontology 2005, 22, 219–226.
- Thomson, W.M.; Lawrence, H.P.; Broadbent, J.M.; Poulton, R. The impact of xerostomia on oral-health-related quality of life among younger adults. Health Qual. Life Outcomes 2006, 4, 86.
- Ikebe, K.; Matsuda, K.; Morii, K.; Wada, M.; Hazeyama, T.; Nokubi, T.; Ettinger, R.L. Impact of dry mouth and hyposalivation on oral health-related quality of life of elderly Japanese. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 216–222.
- Owens, J.; Gibson, B.J.; Periyakaruppiah, K.; Baker, S.R.; Robinson, P.G. Impairment effects, disability and dry mouth: Exploring the public and private dimensions. Health 2014, 18, 509–525.
- Enoki, K.; Ikebe, K.; Matsuda, K.; Yoshida, M.; Maeda, Y.; Thomson, W.M. Influence of xerostomia on oral health-related quality of life in the elderly: A 5-year longitudinal study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 716–721.
- Benn, A.M.L.; Broadbent, J.M.; Thomson, W.M. Occurrence and impact of xerostomia among dentate adult New Zealanders: Findings from a national survey. Aust. Dent. J. 2015, 60, 362–367.
- Thomson, W.M.; Spencer, A.J.; Slade, G.D.; Chalmers, J.M. Is medication a risk factor for dental caries among older people? Evidence from a longitudinal study in South Australia. Community Dent. Oral Epidemiol. 2002, 30, 224–232.
- Grad, H.; Grushka, M.; Yanover, L. Drug induced xerostomia: The effects and treatment. J. Can. Dent. Assoc. 1985, 4, 296–300.
- Handelman, S.L.; Baric, J.M.; Espeland, M.A.; Berglund, K.L. Prevalence of drugs causing hyposalivation in an institutionalized geriatric population. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 26–31.
- Sreebny, L.M.; Schwartz, S.S. A reference guide to drugs and dry mouth—2nd edition. Gerodontology 1997, 14, 33–47.
- Furness, S.; Worthington, H.V.; Bryan, G.; Birchenough, S.; McMillan, R. Interventions for the management of dry mouth: Topical therapies. Cochrane Database Syst. Rev. 2011, 12, CD008934.
- Furness, S.; Bryan, G.; McMillan, R.; Birchenough, S.; Worthington, H.V. Interventions for the management of dry mouth: Non-pharmacological interventions. Cochrane Database Syst. Rev. 2013, 9, CD009603.
- Riley, P.; Glenny, A.M.; Hua, F.; Worthington, H.V. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst. Rev. 2017, 7, CD012744.
- United States Surgeon General. The 2020 Surgeon General’s Report on Oral Health; Department of Health and Human Services: Washington, DC, USA, 2021.
- Geurts, M.M.E.; Talsma, J.; Brouwers, J.R.B.J.; de Gier, J.J. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: A systematic review. Br. J. Clin. Pharmacol. 2012, 74, 16–33.
- Hatah, E.; Braund, R.; Tordoff, J.; Duffull, S.B. A systematic review and meta-analysis of pharmacist-led fee-for-services medication review. Br. J. Clin. Pharmacol. 2014, 77, 102–115.
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern. Med. 2015, 175, 827–834.
- Huiskes, V.J.B.; Burger, D.M.; van den Ende, C.H.M.; van den Bemt, B.J.F. Effectiveness of medication review: A systematic review and meta-analysis of randomized controlled trials. BMC Fam. Pract. 2017, 18, 5.
This entry is offline, you can click here to edit this entry!
