Keratin is a structural protein of mammalian tissues and birds, representing the principal constituent of hair, nails, skin, wool, hooves, horns, beaks, and feathers, and playing an essential role in protecting the body from external harassment. Due to its intrinsic features such as biocompatibility, biodegradability, responsiveness to specific biological environment, and physical-chemical properties, keratin has been extensively explored in the production of nanocarriers of active principles for different biomedical applications.
1. Introduction
The synthesis and discovery of novel and more potent drugs have represented the primary driver of pharmaceutical companies for a long time. Nevertheless, increasing evidence demonstrates that drugs potency is not directly correlated with their therapeutic benefit. In most cases, drug formulation and delivery systems have been revealed to be essential for controlling and improving efficacy, safety, bioavailability, pharmacokinetics, biodistribution, and clearance.
[1] However, effective drug delivery is still challenging primarily because of our limited knowledge of biological barriers and their interaction with drugs’ carriers.
[2] On this basis, enormous efforts have been dedicated to developing suitably designed and effective drug delivery systems based on biomaterials, owing to their biocompatibility, biodegradability, non-immunogenicity, ease of production and up-scaling, and reproducibility.
[3]Among naturally occurring biomaterials, proteins represent one of the most valuable categories for producing drugs nanocarriers due to their abundance, biodegradability, low immunogenicity, non-toxicity, and biocompatibility.
[4] In addition, because of their defined primary structure, the production of protein-based nanocarriers can be properly implemented through pre- or post-functionalization, enabling the covalent attachment of drugs, targeting moieties, dyes, and diagnostic elements, as well as the incorporation of different agents by exploiting the protein hydrophobic and hydrophilic domains.
Among the various proteins used for nanocarrier fabrication, the last decade has witnessed an increasing interest in the use of keratin as a promising and versatile biopolymer for nanoparticle preparation. Keratin is a structural protein present in mammalian tissues (hair, fur, nails, skin, wool, hooves, and horns), and birds (e.g., bird beaks and feathers), playing an important role in protecting the body from external harassment. The keratin content in mammalian hair (such as human hair and wool) and bird feathers exceeds 80%.
[5]Keratin is a cysteine-rich fibrous protein, structurally divided into α-keratins and β-keratins. α-keratins are the primary constituent of soft tissues such as wool, hair, hooves, nails, horns, and the stratum corneum, whereas harder β-keratins are present in feathers, avian claws and beaks, reptilian claws, and scales. The significant presence of disulfide and hydrogen bonds confers to keratin some appealing mechanical properties, such as strength, stability, rigidity, and resistance to proteolytic degradation. Furthermore, keratin is characterized by unique amino acidic sequences, such as “Arg–Gly–Asp” (RGD) and “Leu–Asp–Val” (LDV), that specifically bind vitronectin integrin receptors which, for instance, are overexpressed by several cancer cells. Therefore, keratin could represent a remarkable breakthrough in the field of cancer nanomedicine.
[6]
2. Human Hair Keratin-Based Nanoparticles
Human hair is considered a useless material and usually discarded as waste. In rural and in urban, highly populated areas, tons of hair is either disposed in the land or collected in the waste streams, posing several issues. In fact, due to its composition and slow degradation rate, hair remains for a long period of time, contributing to an increased nitrogen concentration in soil and water. At the same time, if disposed by incineration, hair produces toxic gases such as ammonia, carbonyl sulfides, hydrogen sulfides, sulfur dioxide, phenols, nitriles, pyrroles, and pyridines, that significantly increase pollution.
[7]However, the unique properties of human hair, such as chemical composition, slow degradation rate, high tensile strength, thermal insulation, elastic recovery, and exceptional interaction with water and oils, make it a valuable material for different applications, such as the production of fertilizers, hair extensions, musical instruments, hairbrushes, etc.
[8] Besides the use of human hair per se, keratin, representing the hair’s major constituent, has attracted increasing attention as a biomaterial for biomedical applications.
[9][10]In 2016, Brown et al. studied the use of keratin extracted from hair, under reductive Shindai’s conditions,
[11] that was further covalently functionalized with epigallocatechin (ECGG) through formaldehyde bridges.
[12] The effective EGCG-keratin conjugation was confirmed by the authors through different spectroscopic techniques, e.g., UV-Vis and IR spectroscopy, NMR, and XPS. EGCG-functionalized keratin was then assembled to form spherical nanoparticles, namely NANO, with a hydrodynamic diameter of 50 nm and a polydispersity index of 0.12, as a potential antioxidant and radical scavenging materials. The scavenging ability of NANO was assessed by the authors with two different probes, e.g., 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). At the highest concentration, free EGCG, vitamin C, and NANO showed a comparable DPPH
• scavenging ability (ca. 93%); conversely, the ABTS
•+ scavenging rate of NANO was lower than free EGCG but much higher than vitamin C. Indeed, at a concentration of 7.5 μg/mL, the scavenging rate of NANO, EGCG, and vitamin C was 93.6%, 100%, and 67.1%, respectively. The toxicity of NANO on cell proliferation was evaluated on mouse fibroblast (L929) and on human umbilical vein endothelial (HUVEC) cell lines, showing that they are nontoxic for a concentration of up to 20 mg/mL. The authors also investigated the protective effect of NANO towards macrophages, indicating that nanoparticles efficiently reduce excesses of reactive oxygen species (ROS) by increasing the activity of intracellular antioxidant enzymes, such as superoxide dismutase (SOD), and GSH. Importantly, keratin-EGCG nanoparticles displayed good anti-inflammatory effects by reducing the release of cellular nitric oxide (NO) and pro-inflammatory factors (TNF-α and IL-1β), thus reducing the lipopolysaccharide (LPS)-mediated inflammation. Overall, the work by Li and coworkers demonstrates that human hair keratin-EGCG nanoparticles are promising systems for the protection of cells from inflammation due to imbalanced ROS/NOS production.
In 2020, Gnanamani reported a study where keratin and albumin were used to improve the biocompatibility and storage stability of gold nanoparticles (AuNPs).[13] The authors prepared blank AuNPs, keratin-functionalized AuNPs (KP-AuNPs), albumin-functionalized AuNPs (AP-AuNPs), and keratin-albumin-functionalized AuNPs (KP-APAuNPs) by mixing an aqueous solution of 0.01M HAuCl4·3H2O with the proteins dissolved in water at different concentrations (Figure 1).
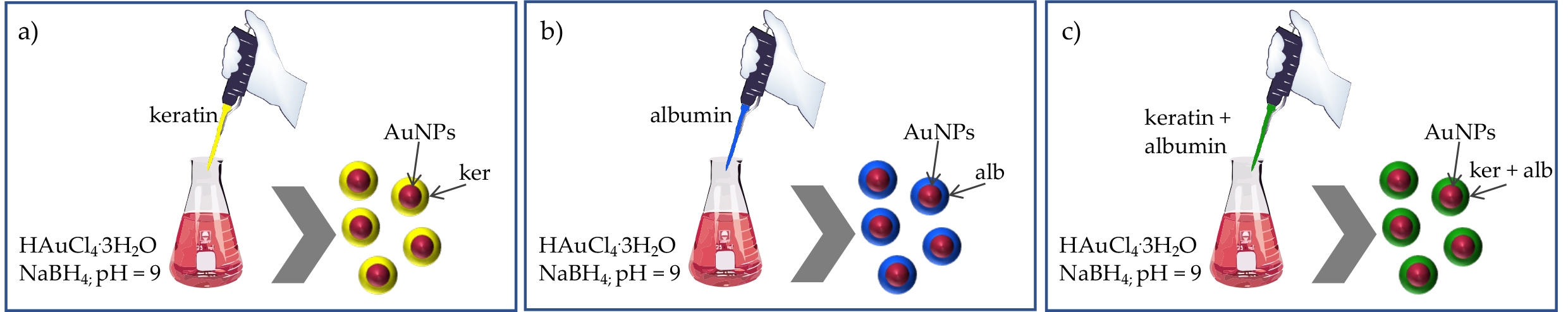
Figure 1. Schematic preparation procedure of (a) KP-AuNPs, (b) AP-AuNPs, and (c) KP-AP-AuNPs.
The successful AuNPs functionalization was confirmed through various characterization techniques, and results revealed that the interaction between the proteins and the AuNPs surface through cysteine and amine groups ultimately determines a significant size increase (from 38 nm to 590 nm). The cytocompatibility, hemocompatibility, and stability, including long-term storage stability (six months), under different pH conditions, were studied and assessed with surface plasmon resonance, suggesting promising properties of protein functionalized nanoparticles as opposed to plain AuNPs. Importantly, results from these authors show that unfunctionalized AuNPs induce an 8% red blood cells lysis, whereas the protein-functionalized particles reached only 2%, and no significant differences were observed in the hemolytic profile of KP-AuNPs, AP-AuNPs, and KP-AP-AuNPs. Overall, the work by Gnanamani and colleagues indicated that keratin alone or in combination with albumin could serve as an effective functional protein for augmenting gold nanoparticles biocompatibility and stability.
3. Wool Keratin-Based Nanoparticles
About two million tons of wool are produced yearly, Australia being the major contributor (over 25%), and wool wastes from textile industries have gained a growing relevance as a renewable source of keratin. Indeed, wool consists of up to 95% of keratin that can be obtained from the hairy part of the hide of sheep, goats, pigs, rabbits, and camelids.
[14] To reduce the environmental impact of textile waste, several keratin extraction procedures have been applied, and the extracted protein has found wide application in the drug delivery field. It is worth noticing that wool-derived keratin seems highly compatible with human biological systems, possessing the same key cell-binding motifs of human keratin that are crucial to support and promote cellular adhesion.
[15]In 2013, Cilurzo et al. demonstrated, for the first time, the feasibility of producing biocompatible keratin-based microparticles starting from wool wastes, and further confirmed particles’ safety on the human monocytic cell line.
[16] In detail, the authors prepared an aqueous solution of regenerated keratin (RK) with a final concentration of 4.5%
w/
w, via sulfitolysis procedure. The FTIR analysis revealed that RK was mainly organized in the disordered α-helix structure, thus resulting in a remarkable water-solubility. After acidic treatment (pH 3.5) followed by washing with water, the RK solution was spray-dried, providing microparticles with a mean diameter of 6 μm, a narrow size distribution, and irregular and smooth surface. Overall, the in vitro biocompatibility assays, performed on human monocytic cell lines at increasing microparticle concentrations (from 1 μg/mL up to 1000 μg/mL for 24 or 72 h), confirmed the particles’ safety. Based on this evidence, various straightforward and water-based procedures have been envisioned which are aimed at obtaining KPNs with tunable morphological, physical, and chemical properties.
As previously mentioned, the sulphitolysis procedure allows for the obtainment of water-soluble and full-length keratin, which, most importantly, preserves the naïve protein primary and secondary structure. In the past few years, several kinds of antitumoral therapeutics (chlorin e6, paclitaxel, doxorubicin, and 9
(R)-9-hydroxystearic acid) have been loaded into keratin nanocarriers. In this regard, our research group described, for the first time, the covalent attachment of the photosensitizer chlorin e6 (ce6) on the keratin backbone via EDCI/NHS coupling, followed by nanoparticle formation, e.g., KNPs@Ce6, by self-assembly and desolvation techniques.
[17] Both the self-assembly and desolvation methods provided stable nanoparticles with comparable hydrodynamic diameters (148 vs. 147 nm) and Ce6 loading ratios (23 vs. 45%), with a monodisperse distribution (PDI: 0.17 vs. 0.13), a negative zeta potential (−53 vs. −68 mV), and with a final yield of 24% and 30%, respectively. The loading of Ce6 on KNPs@Ce6 suspensions (0.1 mg/mL) ranged between 35 and 38 μg/mg of keratin, and was confirmed by UV-vis spectroscopy, displaying a broadened Soret band (400 nm) and a bathochromic shift from 650 nm to 665 nm of the Q-band. After a 24 h incubation period at different concentrations (e.g., 0.5, 2.5, 5.0 μg/mL), KNPs@Ce6 showed a superior internalization compared to free Ce6 on osteosarcoma (U2OS) and glioblastoma (U87) cell lines, and upon light irradiation they produced a large amount of reactive oxygen species (ROS), triggering cell death in a concentration and irradiation-time dependent manner. We further exploited KNPs for delivering paclitaxel (PTX), an anticancer agent widely used in breast and ovarian cancers. KER-NPs-PTX were tested against human breast cancer MCF-7 and MDA MB 231 cell lines in two-dimensional (2D) cultures and in a perfused three-dimensional (3D) model.
[18] Primarily, keratin showed an extraordinary PTX loading ratio up to sevenfold higher as compared to those of albumin, e.g., 43% vs. 6.5% (
w/
w). Moreover, KER-NPs-PTX exerted effective anticancer activities on both 2D and 3D models, leading to a significant increase in the expression of the proapoptotic
BAX gene and cleaved caspase 3 (CC3) protein, and an increment in apoptotic cell percentages, following 24 h treatment. Although monotherapy remains a widely used approach for cancer treatment, its application is strictly limited by severe toxicity, as well as the development of resistance and recurrence phenomena. Therefore, a bimodal therapy combining photo- and chemotherapy could represent an encouraging strategy to overcome these limitations by reducing the effective drug dose and synergistically targeting different pathways.
In this regard, we employed keratin for preparing nanoparticles co-loaded with PTX and Ce6 via drug-induced aggregation and desolvation procedures (PTX-Ce6@kerag and PTX-Ce6@ker
ds) as an innovative approach for the pharmacological treatment of osteosarcoma (OS).
[19] Both methods provided stable nanoparticles with a negative zeta potential (around −46 mV). As expected, PTX-Ce6@ker
ds resulted as smaller and more mono-disperse than PTX-Ce6@ker
ag (hydrodynamic diameter 120 vs. 150 nm, and PDI 0.067 vs. 0.22, respectively), most probably due to the cross-linking mediated by glutaraldehyde. In vitro cytotoxicity assays performed on three osteosarcoma cell lines (MG63, SaOS-2, U-2 OS) indicated that PTX-Ce6@ker
ag displayed a pharmacokinetic profile similar to free PTX, resulting in a significant decrease in cell viability. On the other hand, PTX-Ce6@ker
ds achieved the same cytotoxic effect at higher concentrations; indeed, the crosslinking process may affect the release of PTX, strongly reducing its efficacy. Therefore, PTX-Ce6@ker
ag was selected as the nanoformulation more suitable for further in vitro investigations both in 2D and 3D OS models. Remarkably, cells loaded with PTX-Ce6@ker
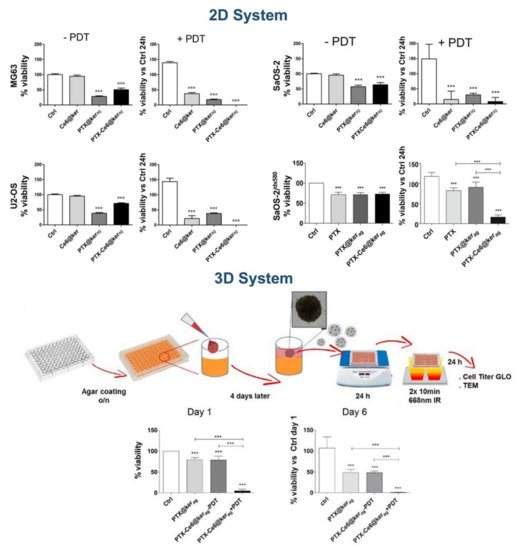
ag, at a final Ce6 and PTX concentration of 3.35 mM and 6.52 mM, respectively, upon light irradiation, showed a dramatic decrease in cell viability, as well as in chemoresistant SaOS-2 cells and in 3D OS models, thus accounting for an additive action of chemotherapy and photoactivation (
Figure 2).
Figure 2. Impact of PTX-Ce6@kerag on 2D and 3D OS tumor model. Reprinted under Creative Common CC BY license from
[19]. ***
p < 0.001.
4. Chicken Feathers Keratin-Based Nanoparticles
Chicken feathers represent the major processed waste product from the poultry industry with around six billion tons of feathers produced annually. Detailed chemical characterization of chicken feathers has been conducted by Tesfaye et al., aimed at ascertaining the possible valorization strategies of these waste products.
[20] The proximate analysis indicated the following composition: crude lipid (0.83%), crude fiber (2.15%), crude protein (82.36%), ash (1.49%), nitrogen free extract (1.02%), and moisture content (12.33%), whereas the ultimate analysis showed: carbon (64.47%), nitrogen (10.41%), oxygen (22.34%), and sulfur (2.64%). Thanks to keratin’s durability and self-extinguishing properties, chicken feathers represent a valuable raw material in several applications, spanning from textile, plastic, cosmetics, pharmaceuticals, biomedical, and bioenergy industries. Furthermore, the keratin fibers could be used as moisture adsorbents, preventing skin from rashes and infections, and as bio-sorbents for treating waste water.
[21]Feather KNPs have been obtained through a plethora of processes, showing tunable and different properties, and making them suitable systems for tissue engineering, wound dressing, and drug carriers.
Pedram Rad et al. obtained a keratin nano-powder by electro-spraying the trifluoroacetic acid solution of recovered feathers protein. This procedure allowed to obtain nanoparticles with tunable diameters, depending on different parameters. The authors observed that the particle size follows a keratin-dependent concentration trend, yielding smaller KNPs at lower polymer concentration (from 24 to 53 nm), as well as increasing the voltage and the nozzle-collector distance, while a higher injection rate led to bigger KNPs. The optimization study allowed for the establishment of the following parameters for obtaining more stable KNPs with an average diameter of 53 nm: keratin concentration = 0.2% (
w/
v), voltage = 15 kV, nozzle-collector distance = 15 cm, feed rate = 0.047 mL/h.
[22]Chicken feather KNPs displayed extraordinary biocompatibility in vitro, as demonstrated by Yang and collaborators on fibroblasts, showing improved cells attachment and proliferation.
[23] As reported, fibroblasts incubated with a medium containing KNPs showed a gradual and continued proliferation for five days, while cells in KNPs-free medium reached a maximum population at days two and three, followed by a steady decrease. These KNPs, prepared by phase separation and ultrasonication methods, showed an average diameter around 70 nm, and, after injection into mice, were found prevalently in kidneys, where 18% of cells were penetrated by nanoparticles.
In 2013, Saravan et al. developed a biodegradable and biocompatible keratin/chitosan-based scaffold for bone tissue engineering applications. In detail, spherical keratin nanoparticles (nKer) with a mean diameter of 150 nm were firstly prepared by the desolvation technique, and then incorporated into a chitosan matrix (CS/nKer). CS/nKer showed a pore size ranging from 17 to 30 μm, which decreases with increasing nKer content; the addition of keratin nanoparticles to the chitosan scaffold produced a slower degradation of the matrix, making it less accessible to lysozyme, despite the fact that no alteration of the swelling behavior was observed. Remarkably, CS/nKer scaffolds exhibited no significant cytotoxicity towards human osteoblastic cells (MG-63), and the addition of nKer significantly improved proteins absorption on the matrix, resulting in a porous architecture that allowed cells and nutrients penetration and tissue ingrowth.
[24] Furthermore, keratin-based carriers displayed other promising biological activities, including hemostatic,
[25] antioxidant, and anticancer efficacies.
[26]Liu and collaborators designed and prepared reduction/pH dual-responsive keratin-based nanoparticles as a delivery system of DOX. In detail, DOX was covalently conjugated onto pegylated keratin through an acid-sensible hydrazone moiety or a reductive cleavable linker, allowing the tumor microenvironment-triggered drug release.
[27][28] Notably, in vitro release studies demonstrated a sustained and cumulative prodrug leakage up to 43% within 107 h in an acidic/reductive environment, while a negligible amount of free DOX was released under physiological conditions. After longer incubation time, prodrug-nanoparticles demonstrated a similar or enhanced antitumor efficacy compared to free DOX in HepG2 human liver and MCF-7 breast cancer cells, probably due to the long-term release in tumor intracellular microenvironment.
Recently, keratin has been also investigated as a platform template for both MRI and therapeutic applications.
[26] Firstly, metal ions (e.g., Mn
2+ and Gd
3+) were sequenced onto the protein’s backbone by exploiting interaction with thiol and carboxyl moieties. Then, a simple and straightforward mixing procedure provided monodisperse metal oxide nanoparticles, MnNPs@Keratin, and GdNPs@Keratin with an average size of ~5 nm and ~8 nm by TEM observation and hydrodynamic diameter of ~12 nm and ~25 nm by DLS analysis. The successful loading of metal ions was confirmed through inductively coupled plasma atomic emission spectrometry (ICP-AES), indicating that the Mn element content in MnNPs@Keratin was 1.3%, and the Gd in GdNPs@Keratin was 3.8%. The
T1 relaxivity (
r1 value) of MnNPs@Keratin and GdNPs@Keratin reached up to 6.8 mM
−1 s
−1 and 7.8 mM
−1 s
−1, respectively, demonstrating concentration-dependent MRI performances. It is worth noting that the
T1-weighted relaxivity of MnNPs@Keratin dramatically increased under acid and reducing conditions, due to the release of Mn
2+ after the reaction between MnO
2 and acids or GSH.
This entry is adapted from the peer-reviewed paper 10.3390/app11209417