Neuroblastoma is one of the most common childhood solid tumors and develops from neural stem cells that normally comprise the embryonic structure termed the neural crest. Human neuroblastoma cell lines have special properties as they exhibit cell growth and are induced to become mature neurons by drugs such as retinoid.
- neuroblastoma
- asymmetric cell division
- centrosome
- MYCN
- TRIM32
- cancer
1. Introduction
Neuroblastoma is one of the most typical solid tumors among childhood cancers and exhibits a wide clinical range [1,2,3]. Patients can be broadly divided into three patterns (low-risk, medium-risk, and high-risk groups) from a biological and clinical point of view [1,2,3]. Although minimum treatment may be sufficient for low-risk patients, high risk patents have poor results in spite of aggressive treatment. Among several biological features, amplification of the MYCN gene is known to correlate with its prognosis and malignancy. In fact, approximately 20% of neuroblastomas have MYCN gene amplification. Recent studies showed that MYCN not only exhibits oncogenic activity, but also plays a role in the self-renewal of normal neural stem cells and progenitor cells [4,5,6,7].
Neuroblastoma is believed to be derived from the multipotent neural crest cells that make up the structure of the embryo [8]. The neural crest is composed of a cell population that produce cell lineage such as Schwann cell, melanocyte, smooth muscle, peripheral neurons, and glia [8]. Therefore, neuroblastoma cells are suspected to be similar in nature to cancer stem cells due to their pluripotent function.
Cancer stem cells are thought to exhibit asymmetric cell division (ACD), which results in tumor cell heterogeneity [9,10]. ACD is a originate physiological process to maintain the stem cell pool and differentiated cell pool through a single cell division. Recent studies showed that the imbalance between self-renewal and differentiation causes the appearance of abnormal stem cells, leading to tumorigenesis in the Drosophila neuroblast population [11,12,13]. Thus, ACD is a strategy for producing many cancer stem cells and differentiated cancer cells.
2. Discovery of ACD in Human Neuroblastoma Cells
ACD studies were originally demonstrated using model organisms, such as nematode embryos [18,19], Drosophila neuroblasts [20], and Drosophila male germ stem cells [21]. These genetic studies showed that the mechanism of ACD is highly conserved. Thereafter, evidence supporting asymmetric cell division in mammalian stem cells, such as muscle [22], skin [23], gut [24], mammary glands [25], the hematopoietic system [26], and developing mouse brain [27,28], was reported.
As mentioned above, human neuroblastoma cell lines have special properties as they exhibit cell growth and are induced to become mature neurons by drugs such as retinoid [29]. In addition, neuroblastoma is a common childhood cancer that may develop at the fetal development stage when a large number of stem cells display ACD [17]. Therefore, we investigate ACD using human neuroblastoma cells as a model [14]. As a result, although the cell lines with MYCN amplification exhibited symmetric cell division (self-renewal division), the cell lines without MYCN amplification exhibited ACD ( Figure 1 ). In addition, comparison of ACD studies using these organisms and model systems demonstrated that ACD in neuroblastoma cells is evolutionarily conserved [14].
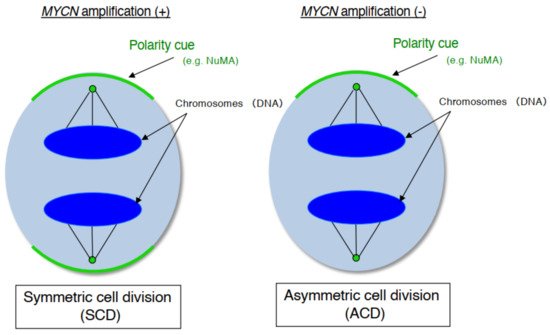
During late mitosis (anaphase), spindle pole-localized NuMA also localizes to the cell cortex as a polarity cue, and the cell cortex localized-NuMA ensures proper polarity division. Neuroblastoma cells with MYCN amplification exhibit symmetric NuMA cortex-based cell division, whereas those without MYCN amplification exhibit asymmetric NuMA cortex-based cell division (the ACD ratio is approximately 20%).
3. MYCN Regulates Cell Division Fate
MYCN is a typical transcriptional factor that belongs to the MYC family (c-MYC, L-MYC) [30]. As mentioned above, recent studies have reported that MYCN not only exhibits oncogenic activity, but also plays a central role in the self-renewal of normal neural stem and progenitor cells [31].
In our experiments, the gene expression level of MYCN influenced the control of cell division fate. The overexpression of MYCN induced symmetric cell division (SCD) (self-renewal division), whereas decreased expression of MYCN caused ACD [14]. Moreover, when its leucine zipper domain was deleted, the MYCN mutant no longer induced self-renewal division. This suggested that the transcriptional activity of MYCN is necessary for inducing SCD in human neuroblastoma cells [14]. Although the specific transcriptional target(s) of MYCN currently remain unclear except for the high mobility group A1 (HMGA1) oncogene [32], several molecular pathways have been identified in MYCN-mediated cell division fate ( Figure 2 ) [17].
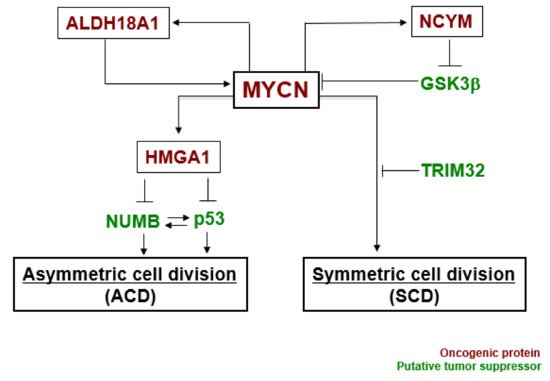
MYCN and ALDH18A1 form a positive feedback loop for their transcriptional expression [33]. This positive feedback loop effectively induces symmetric cell division (SCD). NCYM inhibits GSK3β --mediated phosphorylation of MYCN, resulting in induction of SCD [34,35]. HMGA1 is a transcriptional target of MYCN [32]. HMGA1 inhibits the expression of NUMB [36] and p53 [37]. NUMB and p53 induce asymmetric cell division (ACD) [25,38]. Lastly, TRIM32 facilitates proteasomal degradation of MYCN to induce asymmetric cell division (ACD) [15]. Thus, MYCN-dependent tumor cells exhibit symmetric cell division (SCD) and the degradation of MYCN causes asymmetric cell division (ACD).
4. Induction of ACD in Human Neuroblastoma Cells
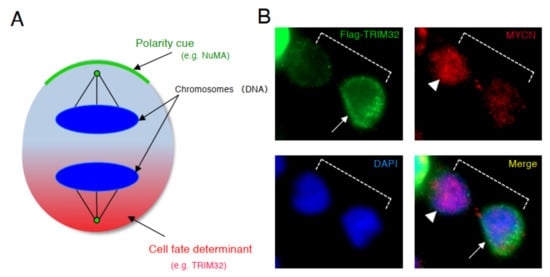
Based on the results of above previous studies, we searched for a molecule that degrades the MYCN protein and induces asymmetric division, focusing on TRIM32 (Tripartite motif-containing 32) by analogy. Previous studies reported that TRIM32 , an ortholog of Drosophila melanogaster , Brat , which controls ACD as a determinant of neuron, suppresses Drosophila MYC (dMYC) function in the neuroblasts of flies [39]. Moreover, mouse TRIM32 was reported to have ubiquitin ligase activity, and facilitated the degradation of the c-MYC oncoprotein during neurogenesis [40]. In neuroblastoma cells, TRIM32 associated with MYCN at the spindle pole during mitosis and facilitated the degradation of MYCN protein as an ubiquitin ligase [15]. In addition, when TRIM32 was overexpressed, ACD was detected in human neuroblastoma cells, as expected ( Figure 3 ) [15].
This entry is adapted from the peer-reviewed paper 10.3390/sym13101907
