Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Schisandra chinensis (Turcz.) Baill. (SCE) is a plant with high potential for beneficial health effects, confirmed by molecular studies. Its constituents exert anti-cancer effects through the induction of cell cycle arrest and apoptosis, as well as inhibition of invasion and metastasis in cancer cell lines and experimental animals. SCE displays antimicrobial effects against several pathogenic strains. It has anti-diabetic potential, supported by hypoglycemic activity. A diet rich in SCE improves pancreatic functions, stimulates insulin secretion, and reduces complications in diabetic animals.
- Schisandra chinensis
- anti-cancer effect
- anti-aging potential
- anti-obesity activity
- anti-diabetic action
1. Introduction
For many years, natural plants have been used in nutrition, food production, and medicine. Many of them contain an array of compounds with antimicrobial, antioxidative, anti-proliferative, and anti-cancer activity [1]. Natural plant compounds have the potential to induce pro-health effects, resulting in an extension of life expectancy and improvement of its quality. Plant extracts and plant-derived compounds can improve the properties of functional food with their well-documented pro-health effects [2].
Schisandra chinensis (Turcz.) Baill. (SCE) is a plant whose fruits have a long-standing use in traditional Chinese medicine. They have been used in the treatment of diseases of the gastrointestinal (GI) tract, respiratory failure, cardiovascular diseases, body fatigue and weakness, excessive sweating, and insomnia [3]. They were also reported to reduce hunger, delay aging, increase vitality, and improve mental health [4]. They demonstrate neuro and hepato-protective, anti-inflammatory, antioxidative, detoxification, immunostimulant, antiviral, and anti-cancer activities, as well as cardiovascular and skin-protective properties [5][6][7][8].
2. Biologically Active Compounds in SCE
S. chinensis contains many bioactive compounds, including lignans, triterpenes, phenolic acids, flavonoids, essential oils, and polysaccharides. Lignans are mainly responsible for the pro-health properties of SCE. These compounds are predominant in SCE fruits, but can also be found in the leaves, shoots, and seeds. They were extracted from the biomass of in vitro cultures [9][10][11]. The most widely represented groups of SCE lignans are dibenzocyclooctadiene lignans, which, due to structural similarity to and occurrence in plants of the Schisandra genus, are often referred to as “schisandra lignans”. Within dibenzocycloactadiene lignans, which occur in the largest amounts in the fruits of Schisandra chinensis, are schisandrin (syn. schisandrol A, wuweizisu A), schisandrin B (syn. gomisin N, wuwezisu B, γ-schisandrin), schisantherin A (syn. gomisin C, schisandrer A), schisantherin B (syn. gomisin B, schisandrer B), schisanhenol (syn. gomisin K3), deoxyschisandrin (syn. schisandrin A), and gomisin A (syn. schisandrol B) [4]. A WHO (World Health Organization) monograph [12] stated that about 30 Schisandra lignans were identified, but to ensure the pro-health activity of fruits, their content should not be lower than 0.4%. At present, many more lignans in SCE have been detected. For example, schineolignins A–C, belonging to the butane-type lignans dibenzyl group, were described by Xue et al. [13]; and schilignan F (tetrahydrofuran lignan) was isolated by Yang et al. [14], from rattan stems of SCE.
The chemical composition and resulting biological activity of plant extracts depends on humidity, light, soil type, latitude, season, maturity, harvest time, geographical location, temperature, and other factors [15]. Additionally, the content of individual lignans in SCE fruits depends on the location of the crop, the degree of fruit maturity, and harvest season [16][17][18]. Zhang et al., (2009) studied ten fruit samples from different provinces of China [16]. In six of them, schisandrin was predominant (2.199–5.332 mg/g), while, in the other four, schisantherin A (2.263–6.36 mg/g) dominated. Thirty fruit samples, examined by Liu et al., showed the highest content of schisandrin (3.51–11.08 mg/g) [17]. This compound constituted 31%–33% of the Schisandra lignans in fruits originating from Korea, and 36%–46% of those from China. In eight out of ten fruit samples, tested by Wang et al., the relationship in the concentration of SCE lignans was schisandrin > gomisin A > schisandrin B [18].
Another important group of biologically active compounds isolated from SCE is the triterpenoids. They constitute a broad and structurally diverse group of chemical compounds. SCE contains lanostane and cycloartane-type triterpenoids and nortiterpenoids, which, in the scientific literature, are often termed as "Schisandra nortriterpenoids" or schinortriterpenoids [19]. An example of a compound belonging to the lanostane-type triterpenoids is kadsuric acid, described by Yang et al. [20]. Examples of cycloartane-type triterpenoids are schisanlactone D and wuweizilactone acid [20][21]. Schinorterpenoids are isolated from different parts of the plant—fruits (schindilactone A, wuweizidilactone I), leaves (schindilactones IK, wuweizidilactones JP, schisanartanin N), rattan stems (schindilactone LM, wuweizidilactone S), and roots (schinchinelactone D) [13][20][22][23][24].
Flavonoids and phenolic acids, which are polyphenols, display antioxidant properties. They are secondary plant metabolites, which occur in every part of the plant (i.e., fruits, flowers, seeds, leaves, roots, or even lignified parts). Among phenolic acids, Mocan et al., found chlorogenic acid in the fruits of SCE, while in the leaves two other derivatives of hydroxycinnamic acid (p-cumaric and ferulic) were found [25]. Significantly more compounds from this group were detected by Szopa et al. [26]. These authors found chlorogenic acid and five hydroxybenzoic acid derivatives: gallic, p-hydroxybenzoic, protocatechuic, syringic, and vanilic acids, in the leaves and fruits. Flavonoids present in SCE fruits are isoquercitin, quercetin, and its derivatives—quercetin 3-galactoside (hyperoside) and quercetin 3-rutinoside (rutin). SCE leaves also contain quercetin 3-ramnoside (quercitrin) myricetin and kaempferol [10][26]. Fruits of SCE also comprise the cyanidin derivatives: Cyanidin-xylosylrutinoside, cyanidin-glucosylrutinoside, cyanidin-xylosylglucoside, and cyanidin-rutinoside, belonging to the anthocyanins [27][28][29].
SCE fruits also contain essential oils. The content of individual groups of compounds can be put in the following order: Sesquiterpene hydrocarbons > oxygenated sesquiterpenes > oxygenated monoterpenes > monoterpene hydrocarbons. The main aromatic compounds are ylangene (11.93%–37.71% of the volatile fraction), α-himachalene (18.03%–20.7%), and β-himachalene (6.29%–10.46%) [30][31].
Finally, polysaccharides isolated from SCE fruits have been intensively studied. SCE is the source of homogeneous polysaccharides composed mainly of glucose, galactose, mannose, and rhamnose in various molar proportions. Their mass ranged from 18 to 127 kDa [32][33][34][35]. Polysaccharides also occur in combination with uronic acid and proteins [36][37][38].
SCE fruits contain substantial amounts of minerals. Sowndhararajan et al., showed that 100 g of dried fruits contains Fe, Mn, Cu, K, and Mg in amounts that cover 96%, 320%, 48%, 54%, and 33% of the Recommended Daily Intake (RDI) of these ingredients, respectively [31]. According to the European Union legal regulations, a food product can be treated as a source of a particular substance if it contains more than 15% of the RDI of that substance in 100 g of the product [39].
3. Health Promoting Effects of SCE Constituents
A rich chemical composition and the presence of diverse biologically active compounds make SCE a plant with strong potential to induce pro-health effects, and its use in disease treatment is subsequently the subject of intense research.
3.1. Antimicrobial Activity
So far, SCE berry extract has demonstrated antibacterial effects against several Gram-positive and Gram-negative bacteria. Oils from SCE seeds showed a good antibacterial activity against Escherichia coli, Bacillus cereus, Enterobacter aerogenes, Serratia marcescens, and Micrococcus luteus, as tested by the disc diffusion method [40]. Teng and Lee [40] investigated the efficacy of various extraction methods, but simultaneous distillation extracted higher amounts of terpenes, β-pinene, borneol, and α-pinene, as well as limonene, than other procedures, including Soxhlet and microwave-assisted extraction. These compounds might display a strong antibacterial activity due to the penetration through the outer membrane of bacterial cells and its severe damage. Six dibenzocyclooctadiene lignans presented antibacterial activity against pathogenic Chlamydia pneumoniae and Chlamydia trachomatis upon their infection in human epithelial cells [41]. The presence and substitution pattern of methylenedioxy, methoxy, and hydroxyl groups of the lignans had a profound impact on the antichlamydial activity [41]. Bai et al., investigated the activity of SCE fruit ethanolic and water extracts against typical food-borne pathogens and food-spoiling organisms [42]. Both extracts displayed strong antibacterial activity towards Staphylococcus aureus, Listeria monocytogenes, Bacillus subtilis, B. cereus, Salmonella enterica subsp. enterica serovar Typhimurium, Pseudomonas aeruginosa, Enterobacter aerogenes and E. coli [42]. It was suggested that the main constituents responsible for such activity were organic acids (such as citric and malic acids), as evaluated by ion chromatography. Mocan et al. evaluated the minimal inhibitory concentration (MIC) of S. chinensis fruit and leaf extracts for the Gram-positive S. aureus, B. subtilis, and L. monocytogenes, and the Gram-negative bacteria E. coli and S. Typhimurium, which ranged from 10 µg/mL to >100 µg/mL [10]. These results indicated that Gram-positive bacteria were more sensitive to SCE extracts than Gram-negative bacteria. The same was observed by Choi et al., for methanol fractions of SCE against several Gram-negative (S. Typhimurium, E. coli, Cronobacter sakazakii) and Gram-positive (B. cereus, L. monocytogenes, S. aureus) strains [43]. This difference may result from the difference in cell wall morphology of these microorganisms [43]. Reports on the stimulation of microbial growth by compounds from SCE are less abundant. In a conference report, S. chinensis rhizome extract was reported to promote growth of Lactobacillus delbrueckii ssp. bulgaricus, while inhibiting activity of Bacillus licheniformis, B. subtilis, and the pathogenic E. coli [44]. The mechanism of inhibition includes changing the permeability of the outer membrane of bacteria, leading to their destruction [44].
3.2. Anti-Cancer Effect
Currently, therapeutics of botanical origin are of high interest in the treatment of cancer and many other diseases. The anti-cancer activity of polyphenols from plant extracts in cancer cell lines includes several mechanisms: Inhibition of tumour proliferation, induction of cell death (apoptotic, autophagic), inhibition of tumour migration and invasion, cell cycle arrest, pro-oxidant activity by stimulation of ROS (reactive oxygen species) production in cancer cell lines, as well as reducing oxidative stress in normal cells and inhibition of carcinogen activity [45]. The main mechanisms of anti-cancer action of SCE phytochemicals are presented in Figure 1.
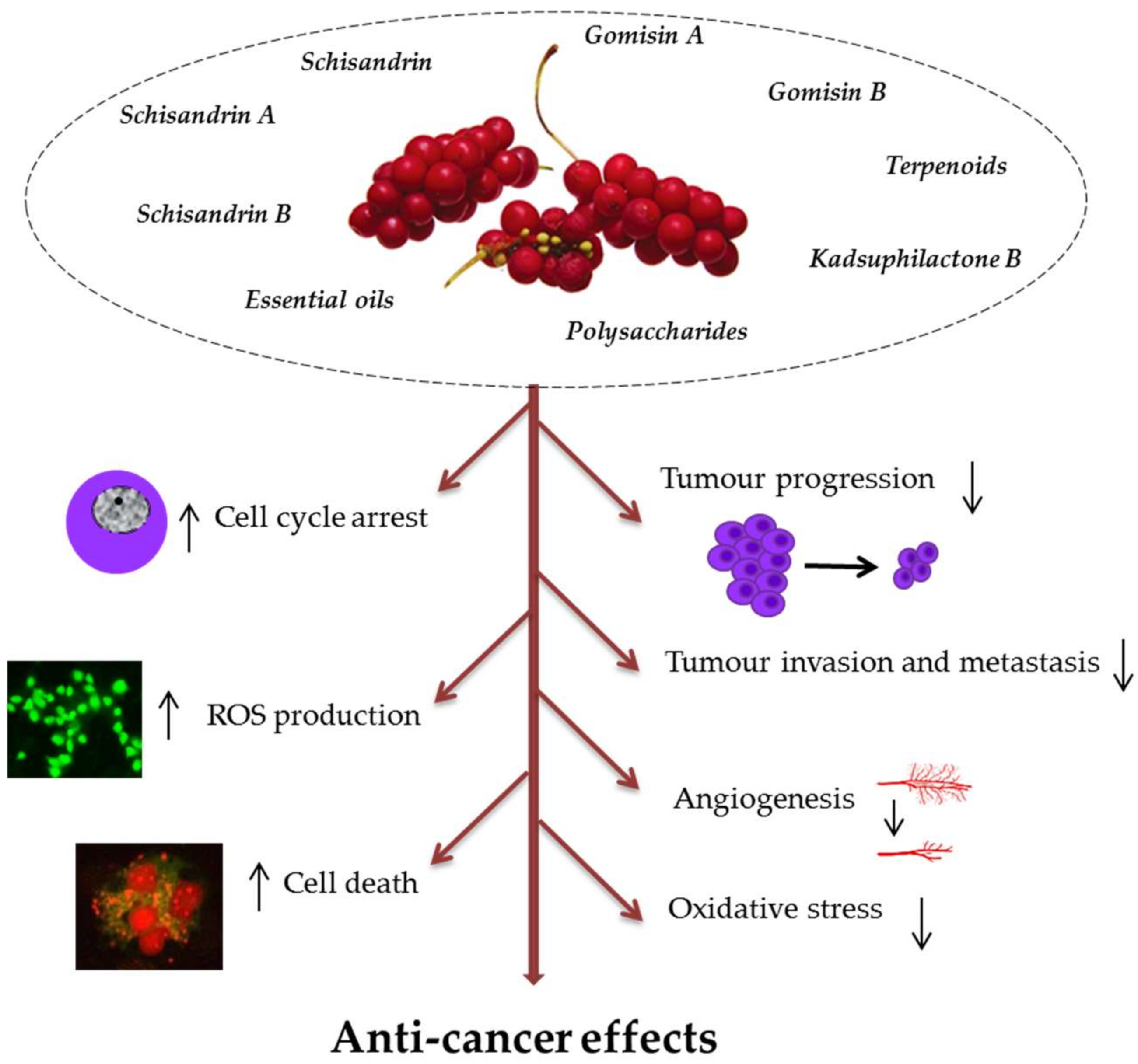
Figure 1. Mechanisms of anti-cancer activity of bioactive phytochemicals in Schisandra chinensis (SCE). They may inhibit tumour progression through cell cycle arrest at G0/G1 and G2/M, suppression of proliferation, invasion, metastasis, and angiogenesis. SCE antioxidative action includes induction of the antioxidant enzymes and direct scavenging of reactive oxygen species (ROS) to prevent cancer induction and progression. Their pro-oxidant effects lead to increased ROS production in cancer cells and cell death (apoptotic and autophagic).
3.3. Anti-Obesity and Anti-Diabetic Action
Due to its antioxidant, hepatoprotective, and anti-cancer activities, SCE fruit has been used as a traditional medicine for treatment of various cardiovascular or GI ailments in South-Eastern Asia and Russia [46]. Recently, the interest in its application as a preventive agent against diet-related chronic diseases, such as type 2 diabetes (T2D), obesity, or non-alcoholic fatty acid disease (NAFLD), has increased.
3.4. Aging-Related Effects of SCE
Organismal aging is determined by many factors besides the date of birth, but cellular senescence can be an important element of aging and age-related diseases [47][48]. Several physiological and pathological conditions are subjects of aging research, including senescence, direct aging, photoaging, oxidative, mitochondrial, and inflammatory aging, among others. To study aging in experimental practice, either cellular replicative senescence is investigated or various animal models of aging are used. D-galactose-induced aging is one (if not the most) commonly applied model of aging [49]. However, it should be considered as a model of accelerated, rather than physiological, aging. It is out of the scope of this review to consider conceptions and models of aging. Instead, some effects of SCE in biological systems, which can be related to aging, will be considered.
Schisandrin B and its analogue, schisandrin C, were shown to protect human and rat foreskin fibroblasts against oxidative damage induced by artificial solar light [50][51]. These substances were proposed to be used in the prevention of skin photoaging. They exerted a protective effect by the stimulation of the production of reduced glutathione, decreased expression of matrix metalloproteinase 1, and an elastase-type protease. However, these compounds also produced ROS during their metabolism, mediated by the cytochrome P-450, and this reaction likely provoked potentiated antioxidant response by the glutathione system. Similar results were obtained for schisandrin B in the human keratinocyte-derived cell line HaCaT [52]. Schisandrin B reduced the cell death, DNA damage, and oxidation of proteins in these cells challenged by oxidative stress; and increased the expression of key enzymes of the antioxidant defence and stimulated the Nrf2 (nuclear factor erythroid 2-related factor 2) and MAPKs (mitogen activated protein kinases) pathways. Similar effects were observed for deoxyschisandrin and schisandrin B in HaCaT keratinocytes exposed to UVB. Altogether, these effects were concluded to be important in the prevention of skin aging underlined by oxidative stress.
Osteoarthritis (OA) is a joint disease, affecting the middle-aged to elderly [53]. An ethanol extract of SCE was shown to exert a protective effect against cartilage degradation in a monosodium iodoacetate (MIA)-induced OA rat model [54]. This protection was underlined by a reduced production of inflammatory cytokines and tumor necrosis factor-alpha (TNFα), an inhibited expression of inducible nitric oxide synthase and cyclooxygenase-2, and increased levels of matrix metalloproteinase-13, cartilage oligomeric matrix protein, and a C-telopeptide of type II collagen.
Sarcopenia, a progressive loss of muscle strength and mass with aging, is commonly considered as an important indicator of normal aging and occurs in some diseases associated with accelerated aging [55]. SCE was shown to increase mass of skeletal muscle in mice and rats treated by dexamethasone or that underwent sciatic neurectomy [56][57][58][59]. To explore the mechanisms beyond these effects, Kim et al. showed that SCE ameliorated muscle atrophy by increased protein synthesis resulting from downregulation of the mTOR/p-4E-BP1 (4E-binding protein1)/p-P70S6K (70 kDa ribosomal protein S6 kinase) pathway in human myoblasts [60]. However, SCE can also promote protein degradation through the FOXO1/MuRF1 pathway, but its net action resulted in muscle hypertrophy. A former work of Kang adds some information on this mechanism, pointing at heme oxygenase-1 (HO-1) and Nrf2, which can be targeted by SCE in C2C12 myoblasts [61]. As aging compromises muscle mass, amelioration of these effects by SCE can be considered as a manifestation of its anti-aging potential. In their recent work, Kim et al. showed that SCE upregulated genes whose products are important in protein synthesis and muscle growth in old mice after chronic forced exercises (swimming) [62]. Additionally, SCE downregulated genes important for protein degradation. SCE also reduced the levels of ROS and lipid peroxidation, as well as upregulating some antioxidant enzymes and inhibited certain apoptotic markers. Therefore, SCE can be considered as an element to assist an exercise-based, healthy life style. Similar conclusions can be drawn from the experiments showing that omija fruit extract as a diet supplement improved the running endurance of rats [56]. That work also showed an upregulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and some other proteins in the skeletal muscle of trained animals.
Gomisin A, another bioactive compound isolated from SCE, was shown to suppress stress-induced premature senescence and the production of proinflammatory molecules in human fibroblasts [63]. This effect was attributed to the promotion of mitochondrial biogenesis and autophagy by gomisin A in these cells, as well as its antioxidant activity. However, some aspects of that work need clarifying, including the determination of the reasons and consequences of the observed effects.
Diet supplementation with schisandrin B was shown to ameliorate age-related impairment of mitochondrial antioxidant functions in various tissues of C57BL/6J mice [64]. This suggests that schisandrin B can increase the survival of aging individuals by improvement of mitochondrial functions. However, despite convincing results on the mutual relationship between aging and antioxidant mitochondrial function in rats, this relationship cannot be directly translated to humans [65].
Rats with accelerated aging, induced by D-galactose, fed with a diet rich in SCE lignans showed the expression of 15 biomarkers of antiaging mechanisms [66]. The markers were involved in energy, amino acid, lipid, and phospholipid metabolism, and almost all returned to the control levels after termination of SCE lignan supplementation. Moreover, a SCE lignan-rich diet resulted in mRNA overexpression of the p19, p53, and p21 proteins in the brain of aging animals. Therefore, these metabolic changes in SCE lignan-fed rats can be underlined by the modulation of the expression of these proteins and become an element of antiaging prevention and therapy.
Aging is not only associated with a decline in biochemical functions, but also in behavioural/cognitive performs [67]. Yan et al., showed that a D-galactose-induced rat, with diet supplemented with ethanol extracts of SCE partitioned with petroleum ether, ameliorated cognitive deficits assayed by the Morris water maze and Step-down type passive avoidance test, as compared to animals with non-supplemented diet [68]. These behavioural changes were associated with a decreased activity of antioxidant enzymes induced by D-galactose and a normal level of oxidative stress markers, including glutathione, malondialdehyde, and nitric oxide in the serum and various structures of the brain of treated animals [68].
In summary, SCE, its extracts, and derivatives can display beneficial effects against pathological aspects of aging in various systems used to investigate aging mechanisms, including cell cultures and animals (Figure 2). How these effects can be related to human aging and age-related diseases remains to be determined, but they justify further research into the anti-aging properties of SCE.
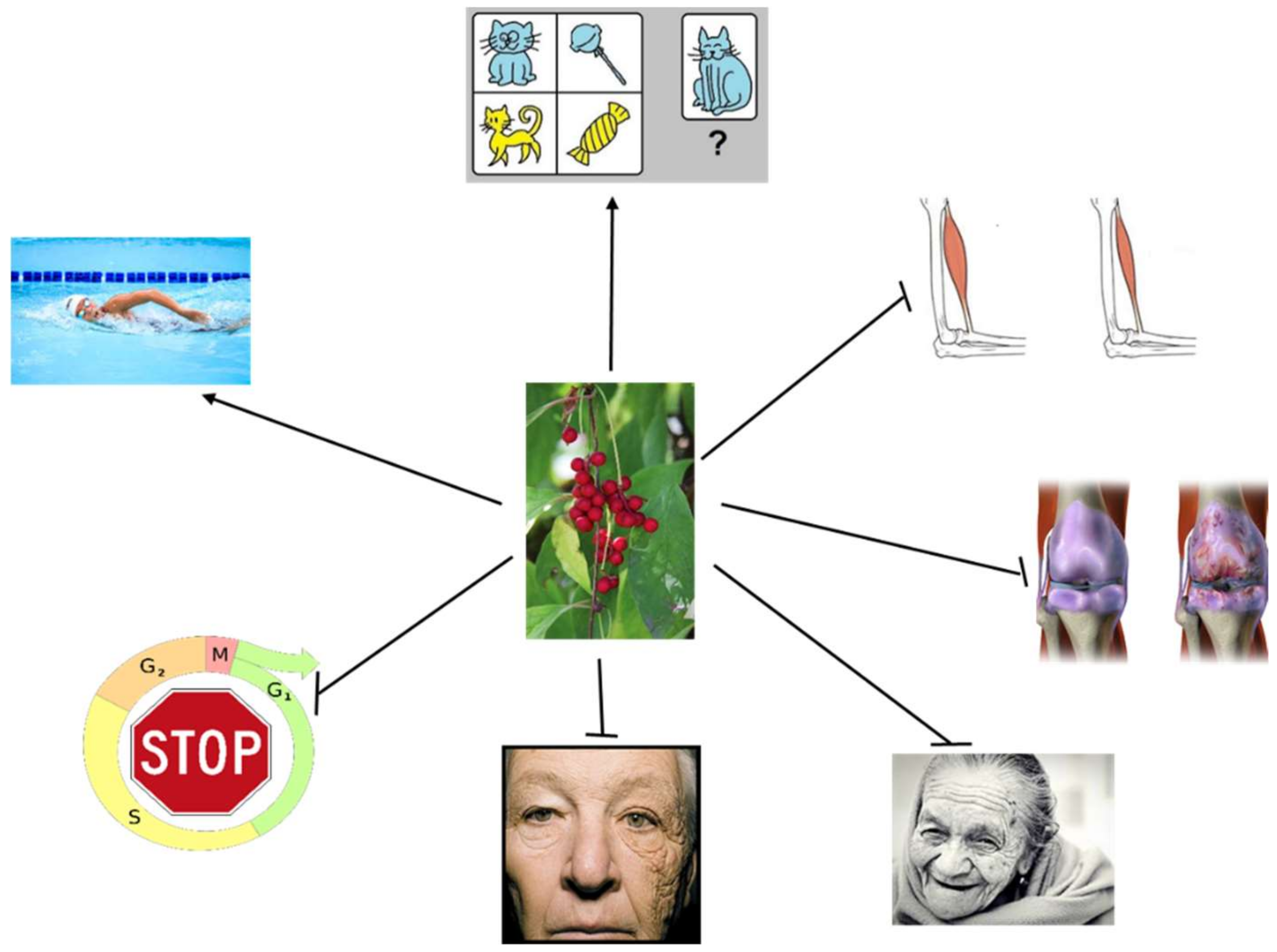
Figure 2. Schisandra chinensis and its derivatives can modulate aging-related phenomena in humans, experimental animals and cell cultures. They can suppress skin photoaging, ameliorate sarcopenia and osteoarthritis, increase physical endurance, inhibit stress-induced premature senescence, improve cognitive and behavioural functions, and modulate other effects that can also be associated with a delay of normal aging.
This entry is adapted from the peer-reviewed paper 10.3390/nu11020333
References
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182.
- Da Silva, B.V.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158.
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212.
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218.
- Zhang, L.; Chen, H.; Tian, J.; Chen, S. Antioxidant and anti-proliferative activities of five compounds from Schisandra chinensis fruit. Ind. Crop. Prod. 2013, 50, 690–693.
- Ranouille, E.; Boutot, C.; Bony, E.; Bombarde, O.; Grosjean, S.; Lazewski, A.; Berthon, J.-Y.; Filaire, E. Schisandra chinensis protects the skin from global pollution by inflammatory and redox balance pathway modulations: An in vitro study. Cosmetics 2018, 5, 36.
- Yang, B.Y.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Li, X.M.; Kuang, H.X. Effects of lignans from Schisandra chinensis rattan stems against Aβ1-42-induced memory impairment in rats and neurotoxicity in primary neuronal cells. Molecules 2018, 23, 870.
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542.
- Ekiert, R.J.; Szopa, A.; Ekiert, H.; Krzek, J.; Dzik, E. Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J. Funct. Foods 2013, 5, 1576–1581.
- Mocan, A.; Crisan, G.; Vlase, L.; Crissan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179.
- Szopa, A.; Ekiert, H. The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill. cultures in vitro. Plant Cell Tissue Organ Cult. 2016, 127, 115–121.
- World Health Organisation. WHO Monographs on Selected Medicinal Plants. 2007. Available online: http://apps.who.int/medicinedocs/en/m/abstract/Js14213e/ (accessed on 2 December 2018).
- Xue, Y.B.; Zhang, Y.L.; Yang, J.H.; Du, X.; Pu, J.X.; Zhao, W.; Li, X.N.; Xiao, W.L.; Sun, H.D. Nortriterpenoids and lignans from the fruit of Schisandra chinensis. Chem. Pharm. Bull. 2010, 58, 1606–1611.
- Yang, B.Y.; Chen, Z.L.; Liu, Y.; Guo, J.T.; Kuang, H.X. New lignan from the rattan stems of Schisandra chinensis. Nat. Prod. Res. 2018, 1–7.
- Inbathamizh, L.; Padmini, E. Effect of geographical properties on the phytochemical composition and antioxidant potential of Moringa oleifera flowers. BioMedRx 2013, 1, 239–247.
- Zhang, H.; Zhang, G.; Zhu, Z.; Zhao, L.; Fei, Y.; Jing, J.; Chai, Y. Determination of six lignans in Schisandra chinensis (Turcz.) Baill. fruits and related Chinese multiherb remedies by HPLC. Food Chem. 2009, 115, 735–739.
- Liu, H.; Lai, H.; Jia, X.; Liu, J.; Zhang, Z.; Qi, Y.; Zhang, J.; Song, J.; Wu, C.; Zhang, B.; et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomedicine 2013, 20, 1135–1143.
- Wang, X.; Yu, J.; Li, W.; Wang, C.; Li, H.; Ju, W.; Chen, J.; Sun, J. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem. Biodivers. 2018, 15, e1800030.
- Xia, Y.G.; Yang, B.Y.; Kuang, H.X. Schisandraceae triterpenoids: A review. Phytochem. Rev. 2015, 14, 155–187.
- Yang, S.; Shan, L.; Luo, H.; Seng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of Schisandra chinensis by UPLC-Q-TOF/MS combined with data post-processing. Molecules 2017, 22, 1778.
- Huang, S.X.; Han, Q.B.; Lei, C.; Pu, J.X.; Xiao, W.L.; Yu, J.L.; Yang, L.M.; Xu, H.X.; Zheng, Y.T.; Sun, H.D. Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Tetrahedron 2008, 64, 4260–4267.
- Shi, Y.M.; Wang, L.Y.; Zou, X.S.; Li, X.N.; Shang, S.Z.; Gao, Z.H.; Liang, C.Q.; Luo, H.R.; Li, H.L.; Xiao, W.L.; et al. Nortriterpenoids from Schisandra chinensis and their absolute configurational assignments by electronic circular dichroism study. Tetrahedron 2014, 70, 859–868.
- Song, Q.-Y.; Gao, K.; Nan, Z.-B. Highly oxygenated triterpenoids from the roots of Schisandra chinensis and their anti-inflammatory activities. J. Asian Nat. Prod. Res. 2016, 18, 189–194.
- Yang, B.Y.; Chen, Z.L.; Liu, Y.; Kuang, H.X. Three new nortriterpenoids from the rattan stems of Schisandra chinensis. Phytochem. Lett. 2018, 24, 145–149.
- Mocan, A.; Zengin, G.; Crisan, G.; Mollica, A. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. 4), 200–210.
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469.
- Ma, C.; Yang, L.; Yang, F.; Wang, W.; Zhao, C.; Zu, Y. Content and color stability of anthocyanins isolated from Schisandra chinensis fruit. Int. J. Mol. Sci. 2012, 13, 14294–14310.
- Liao, J.; Zang, J.; Yuan, F.; Liu, S.; Zhang, Y.; Li, H.; Piao, Z.; Li, H. Identification and analysis of anthocyanin components in fruit color variation in Schisandra chinensis. J. Sci. Food Agric. 2016, 96, 3213–3219.
- Yue, D.; Yang, L.; Liu, S.; Li, J.; Li, W.; Ma, C. A continuous procedure based on column chromatography to purify anthocyanins from Schisandra chinensis by a macroporous resin plus gel filtration chromatography. Molecules 2016, 21, 204.
- Li, X.N.; Cui, H.; Song, Y.Q.; Liang, Y.Z.; Chau, F.T. Analysis of volatile fractions of Schisandra chinensis (Turcz.) Baill. using GC-MS and chemometric resolution. Phytochem. Anal. 2003, 14, 23–33.
- Sowndhararajan, K.; Kim, T.; Kim, H.; Kim, S. Evaluation of proximate composition, bioactive lignans and volatile composition of Schisandra chinensis fruits from Inje and Mungyeong, Republic of Korea. J. Appl. Pharm. Sci. 2016, 6, 001–008.
- Xu, C.L.; Li, Y.H.; Dong, M.; Wu, X.; Wang, X.C.; Xiao, X.S. Inhibitory effect of Schisandra chinensis leaf polysaccharide against L5178Y lymphoma. Carbohydr. Polym. 2012, 88, 21–25.
- Ye, C.; Han, N.; Teng, F.; Wang, X.; Xue, R.; Yin, J. Extraction optimization of polysaccharides of Schisandrae Fructus and evaluation of their analgesic activity. Int. J. Biol. Macromol. 2013, 57, 291–296.
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem. Toxicol. 2013, 55, 609–616.
- Zhao, T.; Feng, Y.; Li, J.; Mao, R.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Chen, Y.; Yang, L.; et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol. 2014, 65, 33–40.
- Tong, H.; Zhao, B.; Du, F.; Tian, D.; Feng, K.; Sun, X. Isolation and physicochemical characterization of polysaccharide fractions isolated from Schisandra chinensis. Chem. Nat. Compd. 2012, 47, 969–970.
- Chi, A.; Zhang, Y.; Kang, Y.; Shen, Z. Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int. J. Biol. Macromol. 2016, 93, 322–332.
- Zhong, S.; Liu, X.D.; Nie, Y.C.; Gan, Z.Y.; Yang, L.Q.; Huang, C.Q.; Lai, K.F.; Zhong, N.S. Antitussive activity of the Schisandra chinensis fruit polysaccharide (SCFP-1) in guinea pigs models. J. Ethnopharmacol. 2016, 194, 378–385.
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. OJ L 404, 30 December 2006; pp. 9–25. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 18 December 2018).
- Teng, H.; Lee, W.Y. Antibacterial and antioxidant activities and chemical compositions of volatile oils extracted from Schisandra chinensis Baill. seeds using simultaneous distillation extraction method, and comparison with soxhlet and microwave-assisted extraction. Biosci. Biotechnol. Biochem. 2014, 78, 79–85.
- Hakala, E.; Hanski, L.; Uvell, H.; Yrjönen, T.; Vuorela, H.; Elofsson, M.; Vuorela, P.M. Dibenzocyclooctadiene lignans from Schisandra spp. selectively inhibit the growth of the intracellular bacteria Chlamydia pneumoniae and Chlamydia trachomatis. J. Antibiot. 2015, 68, 609–614.
- Bai, X.; Park, B.; Hwang, H.-J.; Mah, J.-H. The ability of Schisandra chinensis fruit to inhibit the growth of foodborne pathogenic bacteria and the viability and heat resistance of Bacillus cereus spores. Int. J. Food Sci. Technol. 2015, 50, 2193–2200.
- Choi, E.J.; Jang, S.R.; Kang, O.J.; Bang, W.S. Antimicrobial activity of Psoralea corylifolia, Schisandra chinensis, and Spatholobus suberectus extracts. Korean J. Food Sci. Technol. 2013, 45, 495–500.
- Yu, H. Antimicrobial activity and mechanism of Schisandra chinensis extract. In Proceedings of the 5th International Conference on Environment, Materials, Chemistry and Power Electronics (EMCPE 2016), Zhengzhou, China, 11–12 April 2016; Ankang University: Ankang, China; pp. 192–196.
- Min, K.J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24.
- Liu, H.; Wu, C.; Wang, S.; Gao, S.; Liu, J.; Dong, Z.; Zhang, B.; Liu, M.; Sun, X.; Guo, P. Extracts and lignans of Schisandra chinensis fruit alter lipid and glucose metabolism in vivo and in vitro. J. Funct. Foods 2015, 19, 296–307.
- Jeyapalan, J.C.; Sedivy, J.M. Cellular senescence and organismal aging. Mech. Ageing Dev. 2008, 129, 467–474.
- Campisi, J.; Robert, L. Cell senescence: Role in aging and age-related diseases. Interdiscip. Top. Gerontol. 2014, 39, 45–61.
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251.
- Chiu, P.Y.; Lam, P.Y.; Yan, C.W.; Ko, K.M. Schisandrin B protects against solar irradiation-induced oxidative injury in BJ human fibroblasts. Fitoterapia 2011, 82, 682–691.
- Lam, P.Y.; Yan, C.W.; Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Schisandrin B protects against solar irradiation-induced oxidative stress in rat skin tissue. Fitoterapia 2011, 82, 393–400.
- Ding, M.; Shu, P.; Gao, S.; Wang, F.; Gao, Y.; Chen, Y.; Deng, W.; He, G.; Hu, Z.; Li, T. Schisandrin B protects human keratinocyte-derived HaCaT cells from tert-butyl hydroperoxide-induced oxidative damage through activating the Nrf2 signaling pathway. Int. J. Mol. Med. 2018, 42, 3571–3581.
- Marks, R. Successful aging and chronic osteoarthritis. Medicines 2018, 5, 105.
- Jeong, J.W.; Lee, H.H.; Choi, E.O.; Lee, K.W.; Kim, K.Y.; Kim, S.G.; Hong, S.H.; Kim, G.Y.; Park, C.; Kim, H.K.; et al. Schisandrae Fructus inhibits IL-1β-induced matrix metalloproteinases and inflammatory mediators production in SW1353 human chondrocytes by suppressing NF-κB and MAPK activation. Drug Dev. Res. 2015, 76, 474–483.
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927.
- Kim, Y.J.; Yoo, S.R.; Chae, C.K.; Jung, U.J.; Choi, M.S. Omija fruit extract improves endurance and energy metabolism by upregulating PGC-1 α expression in the skeletal muscle of exercised rats. J. Med. Food 2014, 17, 28–35.
- Kang, J.S.; Han, M.H.; Kim, G.Y.; Kim, C.M.; Chung, H.Y.; Hwang, H.J.; Kim, B.W.; Choi, Y.H. Schisandrae semen essential oil attenuates oxidative stress-induced cell damage in C2C12 murine skeletal muscle cells through Nrf2-mediated upregulation of HO-1. Int. J. Mol. Med. 2015, 35, 453–459.
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42.
- Kim, J.W.; Ku, S.K.; Kim, K.Y.; Kim, S.G.; Han, M.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. Schisandrae Fructus supplementation ameliorates sciatic neurectomy-induced muscle atrophy in mice. Oxid. Med. Cell. Longev. 2015, 2015, 872428.
- Kim, C.H.; Shin, J.H.; Hwang, S.J.; Choi, Y.H.; Kim, D.S.; Kim, C.M. Schisandrae Fructus enhances myogenic differentiation and inhibits atrophy through protein synthesis in human myotubes. Int. J. Nanomed. 2016, 11, 2407–2415.
- Kang, J.S.; Han, M.H.; Kim, G.Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Hyun, Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients 2014, 6, 5667–5578.
- Kim, K.Y.; Ku, S.K.; Lee, K.W.; Song, C.H.; An, W.G. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187.
- Kim, J.S.; Jeong, S.H.; Han, S.H.; Yi, H.K. Gomisin A Modulates aging progress via mitochondrial biogenesis in human diploid fibroblast cells. Clin. Exp. Pharmacol. Physiol. 2018, 45, 547–555.
- Ko, K.M.; Chen, N.; Leung, H.Y.; Leong, E.P.; Poon, M.K.; Chiu, P.Y. Long-term schisandrin B treatment mitigates age-related impairments in mitochondrial antioxidant status and functional ability in various tissues, and improves the survival of aging C57BL/6J mice. BioFactors 2008, 34, 331–342.
- Meng, Q.; Wong, Y.T.; Chen, J.; Ruan, R. Age-related changes in mitochondrial function and antioxidative enzyme activity in Fischer 344 rats. Mech. Ageing Dev. 2007, 128, 286–292.
- Sun, J.; Jing, S.; Jiang, R.; Wang, C.; Zhang, C.; Chen, J.; Li, H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by d-galactose. Clin. Interv. Aging 2018, 13, 829–841.
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J. Chem. Neuroanat. 2019, 95, 70–80.
- Yan, T.; Shang, L.; Wang, M.; Zhang, C.; Zhao, X.; Bi, K.; Jia, Y. Lignans from Schisandra chinensis ameliorate cognition deficits and attenuate brain oxidative damage induced by d-galactose in rats. Metab. Brain Dis. 2016, 31, 653–661.
This entry is offline, you can click here to edit this entry!
