Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Horticulture
Increasing temperature leads to intensive water evaporation, contributing to global warming and consequently leading to drought stress. These events are likely to trigger modifications in plant physiology and microbial functioning due to the altered availability of nutrients. Plants exposed to drought have developed different strategies to cope with stress by morphological, physiological, anatomical, and biochemical responses.
- climate change
- drought stress
- biopreparations
- plant stimulation
- plant growth-promoting microorganisms
1. Introduction
The horticulture system is affected by various abiotic and biotic stresses which directly and indirectly influence soil fertility, plant health and crop yield [1][2][3]. These stresses result in the loss of soil microbial diversity, soil fertility and availability of nutrients [4]. The condition of the soil under drought strictly corresponds to plant performance, showing consequences in plant morphology, anatomy, physiology, and biochemistry. With reduction in seed germination and seedling growth, plant height, nutrition and biomass are weakened resulting in yield limitation. The huge variety of changes taking place in horticultural plants and the mechanisms of counteracting stress they produce result from a very wide range of horticultural plant species, including types of crops such as those distinguished by the International Society for Horticultural Science (ISHS): (1) tree, bush and perennial fruits, (2) perennial bush and tree nuts, (3) vegetables (roots, tubers, shoots, stems, leaves, fruits and flowers of edible and mainly annual plants), (4) medicinal and aromatic plants, (5) ornamental plants, (6) trees, shrubs, turf and ornamental grasses propagated and produced in nurseries for use in landscaping or for establishing fruit orchards or other crop production units [5]. Facing the current, rapid climate changes, the cultivation of plants is strongly affected by abiotic stresses, which additionally intensify the influence of biotic factors such as pests causing serious plant infections [4]. In this dramatic situation, plant associations with rhizospheric [6][7] and endophytic [8][9] microorganisms colonizing the rhizoplane, rhizosphere and plant tissues should be considered as the main stress relievers [10][11][12][13][14]. Three types of effects of microorganisms associated with plants are distinguished: beneficial, deleterious and neutral ones. Based on the positive effects of microbes, two main groups are listed, plant growth-promoting rhizobacteria (PGPR) or more generally, plant growth-promoting bacteria (PGPB) and plant growth-promoting fungi (PGPF) [14][15][16][17][18][19]. All mentioned groups of microorganisms can serve as biocontrol agents, biofertilizers, phytostimulators and phytoremediators [2][12][20][21][22].
The most frequently described biochemical mechanisms of plant protection against drought by microorganisms are the production of phytohormones, antioxidants and xeroprotectants [23]. Trehalose can act as xeroprotectant triggering the plant-defense system to counteract the damage caused by drought. It has been shown that microorganisms with tolerance to desiccation have the ability to protect some plants from drought. It seems to be dependent on the microorganism’s ability to regulate the concentration of trehalose in the plant as a signal of drying damage.
In horticultural production, plant–microbe interactions should be considered the main factor of plant growth, protection against abiotic stresses and resistance against adverse conditions [24][25] (e.g., in arid and semiarid areas), and these interactions could also be beneficial in alleviating drought stress in plants [26]. Profound knowledge about the mechanisms of plant–microbe interactions can offer several strategies to increase plant productivity in an environmentally friendly manner [27]. Therefore, in the increasing market for plant growth-promoting products, it is important to develop a successful strategy for microorganism screening [28]. Furthermore, the European Green Deal (EGD), provided by the European Commission in December 2019, is currently focused on the application of natural products in agriculture and horticulture instead of chemical plant-protection products. To cope with this idea, new efficient biological ingredients in the face of changing climate are desired. Nowadays, the most significant consequence of climate change is drought stress [29].
To deal with severe drought stress in the near future, it is strictly necessary to determine the interactions, mechanisms and signaling pathways responsible for increased drought tolerance in terrestrial organisms. The concept of drought and water deficit is difficult to define, but the literature data [30][31][32] indicate that drought can be defined as a state of the total water capacity being within the range of 12–20% for a period of 16 days. Moreover, the drought state can achieve at least two degrees—mild and severe [33]—while the water deficit [34] refers to the state of water capacity falling below 30%. To handle the drought effect, plants can be supported by both microorganisms inhabiting the rhizoplane (i.e., those adhering to the surface of the roots) and rhizosphere (i.e., living at a further distance within the root secretions) [34][35], as well as endophytic microorganisms inhabiting the inside of the root [36].
2. Climate Change
Global climate change is expected to be considerably critical over the century, leading to influences on various parameters of the environment [17]. Not only atmospheric CO2 concentrations derived from natural and anthropogenic sources, but also surface temperatures will be increasing gradually, likely from 1.0 to 5.7 °C by the end of this century [37]. Moreover, some regions, such as the Eastern Mediterranean and Middle East (EMME), have been classified as a climate “global hot-spot”. In the EMME, the temperature is predicted to increase from 3.5 to 7 °C by the end of the century [38]. Additionally, it is anticipated that rising air temperatures will increase the frequency of extreme weather disasters such as heat waves, drought and heavy precipitation occurrence to a level that has never been monitored before [37]. These strongly temperature-dependent climate changes, combined with water scarcity, will lead to enhanced drought throughout the globe, hurting whole ecosystems and different organisms, including the distribution of plants and microorganisms [17].
In climate studies, calculations concerning crop evapotranspiration are also important [17]. For instance, in South East Europe, the mean annual crop evapotranspiration in the period 1991–2020 reached from 56 mm to 1297 mm, while averages for the future 30 years (between 2021 and 2050), are expected to vary from 59 mm to 1410 mm [17]. These predictions consider the impact of future climate warming. Global warming increases water evaporation and consequently leads to drought stress [39]. High temperature is the crucial factor in melting glaciers and increasing the sea level [8]. The changes in polar and subpolar climate zones also correspond with climate warming [40][41][42].
Climate change results in altered environmental conditions and negative effects on natural ecosystems, which are likely to trigger modifications in plant physiology [43] and microbial functioning [44] based on the availability of nutrients [4] or signal compounds [2]. It is certain that not only plants, but also plant-associated microorganisms might be remarkably changed in abundance, diversity and activity [44][45]. Both increased temperature and drought may activate correspondent adjustments in plants and microorganisms and their mutual interactions [17]. The adaptational challenges of horticultural plants are not only associated with long-term average climate change, but also with the short-term changes driven by weather extremes and interannual fluctuations [46]. Drought-related cereal production losses are increasing by more than 3% yr−1 [46]. In the face of the continuous raise of the world population to an estimated nine billion by 2050 [47], withstanding drought stress according to sustainable agriculture/horticulture is a challenge for the 21st century [48].
3. Plants under Drought Stress
Drought is an uncontrolled stress which affects almost all stages of plant growth and development directly or indirectly [43]. Most of the drought effects on plants are associated with high temperature. Physiological processes occur mostly in temperatures ranging from 0 °C to 40 °C. However, the optimal temperatures for the different stages of growth and development are narrower and strongly depend on the species and ecological origin [1][49].
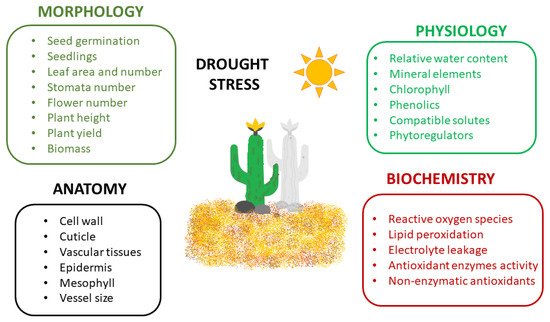
Plants exposed to drought stress develop numerous responses in different areas, from morphological and physiological mechanisms to anatomical and biochemical or molecular ones [1][39][50] (Figure 1).
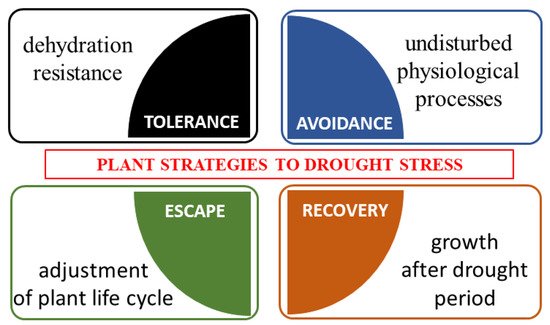
Four types of morphological and physiological response strategies to drought stress are highlighted, i.e., tolerance, avoidance, escape and recovery [51] (Figure 2). Tolerance is defined as the plant’s ability to resist dehydration using osmoprotectants [52]. Avoidance is based on the undisturbed occurrence of physiological processes (such as stomata regulation, root system development). Escape is the adjustment of the plant’s life cycle by shortening of the life cycle to avoid drought stress. Recovery is the ability of a plant to restart growth after the exposure to the extreme drought stress [53].
The morphological features of drought stress include limited seed germination and seedling growth, reduced size, area and number of leaves, restricted number of stomata, reduced number of flowers, disturbed stem and root elongation, impaired plant height, growth, development and yield, and reduced fresh and dry biomass [7][39][50].
In order to adapt to the adverse environment, avoid drought and improve water availability, plants increase the root length and their number [54]. Drought significantly affects the plant’s cell elongation and division, its growth and its development, which is mainly caused by the reduction in cellular differentiation, plant growth and yield [50]. The negative effect on the leaf area under the drought condition could be dependent on the reduction in the leaf number, size and longevity, combined with temperature, leaf turgor pressure and assimilation rate [55]. The reduction in plant height and shoot dry weight results in a lower quality of yield [54].
The morphological responses are most frequently combined with anatomical changes in plants exposed to drought, e.g., thickening of cell walls, increased cuticle layer on the leaf surface and improved development of vascular tissues [8][56]. Drought stress results in anatomical changes in the lower and upper epidermis, mesophyll tissue and vascular bundle diameter of leaves [57]. The negative anatomical effects on the leaves are based on a shortage of water supply from the soil, limitations in nutrients uptake, and reduction in photosynthetic rate. Plant hydraulic conductivity is modulated during drought stress leading to the disruption of water flow in the xylem vessels (embolism) or modifications in the vessel size and function [58]. Consequently, the reduced water flow from the root to the shoot causes stomatal closure and transpiration disruption [50].
Drought affects the physiological traits such as the leaf relative water content and water potential, stomatal conductance, transpiration and photosynthetic rates [59][60]. Reduced water content and water conductivity are responsible for the loss of turgidity and limited stomatal conductance resulting in restricted gaseous exchange (the rate of carbon assimilation) [8][61]. Furthermore, climatic conditions, e.g., higher temperature, drought and soil aeration reduce the movement of nutrients in the soil, their uptake by roots and transport in plant tissues [62].
Photosynthesis can be disrupted through the modulation of the electron transport chain and can increase the rate of biochemical reactions catalyzed by different enzymes. Above a certain temperature threshold, enzymes lose their function, influencing the plant tissue tolerance to drought [1][63][64]. Drought stress also affects the translocation of nutrients and the composition of minerals, antioxidants and proteins [39][52]. Under stress conditions, reactive oxygen species (ROS) are highly generated [65][66] causing cell damage and plant necrosis [67]. Additionally, plant hormones and primary and secondary metabolites are modified [1]. Drought is the elicitor that can increase the content of secondary metabolites in plant tissues such as flavonoids, phenolics or more specific molecules, e.g., glycosides and alkaloids [68][69].
Crosstalk between drought and salinity stresses results in secondary stresses such as oxidative and osmotic ones [66]. Drought stress is a major agricultural problem worldwide and almost all of the main agricultural lands are affected by drought stress. The potential mechanisms of drought tolerance include: (1) production of phytohormones (such as indole-3-acetic acid (IAA), cytokinins and abscisic acid (ABA)) (2) synthesis of exopolysaccharides (3) activity of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase (4) induced systemic tolerance [66][70].
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae7100390
References
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832.
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856.
- Sousa, C.S.; Soares, A.C.F.; Garrido, M.S. Characterization of Streptomycetes with potential to promote plant growth and biocontrol. Sci. Agric. 2008, 65, 50–55.
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 1–22.
- International Society for Horticultural Science. Available online: https://www.ishs.org/defining-horticulture (accessed on 27 September 2021).
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant-microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406.
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551.
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397.
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340.
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-microbe interactions facing environmental challenge. Cell Host Microbe 2019, 26, 183–192.
- Kavadia, A.; Omirou, M.; Fasoula, D.; Ioannides, I.M. The importance of microbial inoculants in a climate-changing agriculture in Eastern Mediterranean region. Atmosphere 2020, 11, 1136.
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-treated seedlings under sustainable agriculture: A global perspective facing climate change. Agronomy 2021, 11, 14.
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2020, 34, 2075–2086.
- Fincheira, P.; Quiroz, A.; Tortella, G.; Diez, M.C.; Rubilar, O. Current advances in plant-microbe communication via volatile organic compounds as an innovative strategy to improve plant growth. Microbiol. Res. 2021, 247, 126726.
- Hanaka, A.; Ozimek, E.; Majewska, M.; Rysiak, A.; Jaroszuk-Ściseł, J. Physiological diversity of Spitsbergen soil microbial communities suggests their potential as plant growth-promoting bacteria. Int. J. Mol. Sci. 2019, 20, 1207.
- Hanaka, A.; Nowak, A.; Plak, A.; Dresler, S.; Ozimek, E.; Jaroszuk-Ściseł, J.; Wójciak-Kosior, M.; Sowa, I. Bacterial isolate inhabiting Spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. Int. J. Mol. Sci. 2019, 20, 1909.
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214.
- Ma, Y.; Vosátka, M.; Freitas, H. Editorial: Beneficial microbes alleviate climatic stresses in plants. Front. Plant Sci. 2019, 10, 595.
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192.
- Gupta, S.; Seth, R.; Sharma, A. Plant Growth-Promoting Rhizobacteria Play a Role as Phytostimulators for Sustainable Agriculture; Choudhary, D., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016.
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918.
- Pirttilä, A.M.; Tabas, H.M.P.; Baruah, N.; Koskimäki, J.J. Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms 2021, 9, 817.
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577.
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163.
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172.
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276.
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the omics of plant-microbe interaction: Perspectives and new insights. Curr. Genom. 2020, 21, 343–362.
- Vasseur-Coronado, M.; du Boulois, H.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 1–10.
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616.
- Kränzlein, M.; Geilfus, C.-M.; Franzisky, B.L.; Zhang, X.; Wimmer, M.A.; Zörb, C. Physiological responses of contrasting maize (Zea mays L.) hybrids to repeated drought. J. Plant Growth Regul. 2021.
- Jamil, M.; Ahamd, M.; Anwar, F.; Zahir, Z.A.; Kharal, M.A.; Nazli, F. Inducing drought tolerance in wheat through combined use of L-tryptophan and Pseudomonas fluorescens. Pak. J. Agric. Sci. 2018, 55, 331–337.
- Yin, B.; Wang, Y.; Liu, P.; Hu, J.; Zhen, W. Effects of vesicular-arbuscular mycorrhiza on the protective system in strawberry leaves under drought stress. Front. Agric. China 2010, 4, 165–169.
- Hone, H.; Mann, R.; Yang, G.; Kaur, J.; Tannenbaum, I.; Li, T.; Spangenberg, G.; Sawbridge, T. Profiling, isolation and characterisation of beneficial microbes from the seed microbiomes of drought tolerant wheat. Sci. Rep. 2021, 11, 11916.
- Zapata, T.; Galindo, D.M.; Corrales-Ducuara, A.R.; Ocampo-Ibáñez, I.D. The diversity of culture-dependent gram-negative Rhizobacteria associated with manihot esculenta crantz plants subjected to water-deficit stress. Diversity 2021, 13, 366.
- Mustafa, S.; Kabir, S.; Shabbir, U.; Batool, R. Plant growth promoting rhizobacteria in sustainable agriculture: From theoretical to pragmatic approach. Symbiosis 2019, 78, 115–123.
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7.
- IPCC Climate Change. The Physical Science Basis 2021; IPCC Climate Change: Geneva, Switzerland, 2021.
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Chang. 2012, 114, 667–687.
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944.
- Hanaka, A.; Plak, A.; Zagórski, P.; Ozimek, E.; Rysiak, A.; Majewska, M.; Jaroszuk-Ściseł, J. Relationships between the properties of Spitsbergen soil, number and biodiversity of rhizosphere microorganisms, and heavy metal concentration in selected plant species. Plant Soil 2019, 436, 49–69.
- Mędrek, K.; Gluza, A.; Siwek, K.; Zagórski, P. The meteorological conditions on the Calypsobyen in summer 2014 on the background of multiyear 1986–2011. Probl. Klim. Polar. 2014, 24, 37–50. (In Polish)
- Franczak, Ł.; Kociuba, W.; Gajek, G. Runoff variability in the Scott River (SW Spitsbergen) in summer seasons 2012–2013 in comparison with the period 1986–2009. QuaGeo 2016, 35, 39–50.
- Hu, Y.; Xie, G.; Jiang, X.; Shao, K.; Tang, X.; Gao, G. The relationships between the free-living and particle-attached bacterial communities in response to elevated eutrophication. Front. Microbiol. 2020, 11, 423.
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432.
- Manzanera, M. Dealing with water stress and microbial preservation. Environ. Microbiol. 2021, 23, 3351–3359.
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jagermeyr, J. Severity of drought and heatwave crop losses tripled over the last five decades in Europe. Environ. Res. Lett. 2021, 16, 065012.
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515.
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687.
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High temperature alters secondary metabolites and photosynthetic efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756.
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520.
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689.
- Luo, W.; Xu, C.; Ma, W.; Yue, X.; Liang, X.; Zuo, X.; Knapp, A.K.; Smith, M.D.; Sardans, J.; Dijkstra, F.A.; et al. Effects of extreme drought on plant nutrient uptake and resorption in rhizomatous vs bunchgrass-dominated grasslands. Oecologia 2018, 188, 633–643.
- Manavalan, L.P.; Guttikonda, S.K.; Phan Tran, L.S.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276.
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225.
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202.
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211.
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630.
- Hargravei, K.R.; Kolb, K.J.; Ewers, F.W.; Davis, S.D. Conduit diameter and drought-induced embolism in Salvia mellifera Greene (Labiatae). New Phytol. 1994, 126, 695–705.
- Liu, F.; Jensen, C.R.; Shahanzari, A.; Andersen, M.N.; Jacobsen, S.E. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836.
- Ullah, A.; Mushtaq, H.; Fahad, S.; Hakim; Shah, A.; Chaudhary, H.J. Plant growth promoting potential of bacterial endophytes in novel association with Olea ferruginea and Withania coagulans. Microbiology 2017, 86, 119–127.
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393.
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281.
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7.
- Zhang, Y.B.; Yang, S.L.; Dao, J.M.; Deng, J.; Shahzad, A.N.; Fan, X.; Li, R.D.; Quan, Y.J.; Bukhari, S.A.H.; Zeng, Z.H. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 2020, 15, e0235845.
- Cruz de Calvadio, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165.
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. Reactive oxygen, nitrogen and sulfur species in plants: Production, metabolism, signaling and defense mechanisms. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 311–336.
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 1–16.
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 1–10.
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771.
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14.
This entry is offline, you can click here to edit this entry!