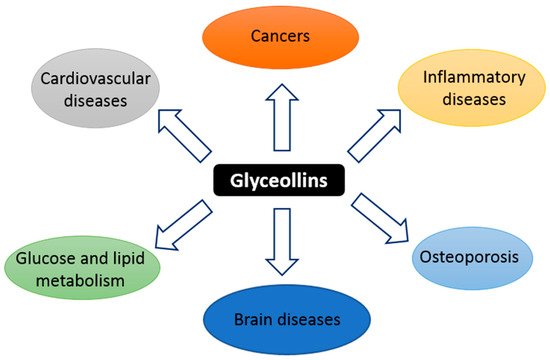
Glyceollins are a group of phytoalexins that are isolated from soybeans. They have attracted attention because they exert numerous effects on human functions and diseases, notably anticancer effects.
1. Introduction
Thousands of bioactive molecules, among phytochemicals, naturally occur in plants after secondary metabolism. Out of these compounds, polyphenols represent the most important family, including more than 500 identified compounds [
1,
2]. They are mainly composed of flavonoids, phenolic acids, stilbenes, and lignans, found in a wide variety of plant-based foods. Polyphenol concentrations in foods and quantities consumed differ considerably, leading to wide differences in the daily intake between polyphenol compounds [
2]. Polyphenols have often been associated with a decreased incidence of several human diseases and have been used in traditional medicines and functional foods [
3]. Epidemiological studies have shown that a high intake of polyphenolic phytochemicals in the diet can prevent many diseases, such as cancers, diabetes or inflammatory, cardiovascular, and neurodegenerative diseases [
4,
5,
6,
7,
8,
9]. However, these studies have some limitations because they do not always take into account the geographical diversity, sex, age, and region of residence of the subjects. In addition, an accurate evaluation of polyphenol intake, which appears to be essential in these studies, is not always completed [
2,
8]. Polyphenols are also used as food supplements and in pharmaceutical and cosmetic products [
10,
11,
12]. One of the advantages of ingesting phytochemicals through the diet is that combinatory effects from these compounds can occur [
9].
The phytoalexins, a group of polyphenolic compounds possessing strong antimicrobial and antifungal properties, are produced by plants as defense molecules against phytopathogens [
13]. They are only present in very small quantities in healthy plants, but accumulate in large quantities following an attack by bacteria, fungi, or nematodes [
14,
15]. In addition to biotic agents, other stresses, such as high temperature, ultraviolet (UV) radiation, humidity, and dryness, can also induce the production of these compounds [
14,
15]. Phytoalexins are very diverse and have been found in various crops, such as rice [
16], soybean [
17], maize [
18], barley [
19], and banana [
20]. Phytoalexins can be produced in numerous parts of the plant, such as the flowers, leaves, stems, seeds, and root tubers [
19,
21].
Glyceollins, a group of phytoalexins, are the most important bioactive compounds present in soybeans upon exposure to certain fungi and some abiotic elicitors, such as UV light, aluminum chloride, or methyl jasmonate [
17,
22]. Glyceollin I, II, and III (GI, GII, and GIII) are de novo synthesized from the soy isoflavone daidzein. In addition to their antibacterial, antifungal, and antinematode actions, glyceollins have recently received much attention because of their antiproliferative, antiestrogenic, anti-inflammatory, antioxidative, and anticholesterolemic activities. They may possess potential medicinal properties in humans, notably protective effects against hormone-dependent cancers, and metabolic and cardiac diseases (
Figure 1) [
23,
24]. Accordingly, glyceollins are effectively able to inhibit proinflammatory cytokines by inhibiting the activation/phosphorylation of the transcription nuclear factor-kappa B (NF-κB). They prevented the lipopolysaccharide (LPS)-induced expression of nitric oxide synthase (iNOS) and cyclo-oxygenase (COX)-2 in murine macrophage cell lines and were able to reduce 12-
O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation in mice. These natural compounds also inhibit hormone-dependent breast and ovarian cancer cell growth [
25,
26]. One of the mechanisms responsible for the antitumoral effect of glyceollins is their antiestrogenic action [
25,
27,
28]. However, other antitumorigenic actions, whose mechanisms are not well understood, of glyceollins have also been reported. For instance, Lee et al. reported on an inhibitory effect of glyceollins on the kinase activity of the vascular endothelial growth factor (VEGF) receptor as well as its downstream signal transduction pathways involved in angiogenesis and tumor growth [
29]. Likewise, an inhibitory effect on the expression of hypoxia inducible factor 1 (HIF-1)-target genes (such as VEGF) in cancer cells was described [
30]. In addition, glyceollins were able to inhibit phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR ) pathways involved in the control of HIF-1 expression in solid tumor tissues. Moreover, a study by Carriere et al. showed that the epithelial-mesenchymal transition of breast cancer cells that are resistant to aromatase inhibitors can be blocked by GI [
31]. This effect is partially mediated by the inhibition of zinc finger E-box binding homeobox 1 (ZEB1) expression.
Figure 1. Schematic summary of the targets of glyceollins.
2. Synthesis and Structure
Previously described as hydroxyphaseollin in the early 70s [
32,
33], glyceollins belong to the family of phytoalexins, which are antimicrobial agents in plants. Glyceollins were characterized in soybeans after inoculation with the pathogen agent
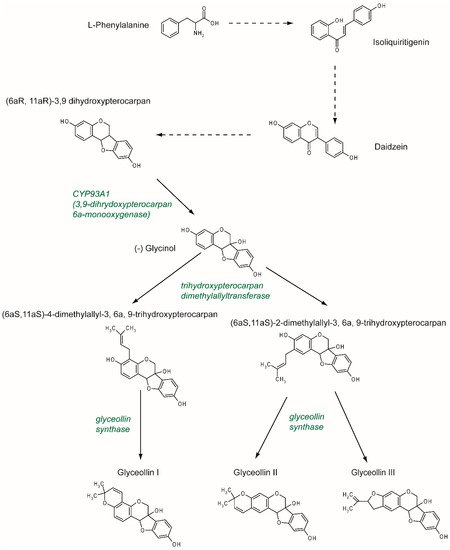
Phytophthora sojae. There are at least five glyceollins described to date that are produced via a complex metabolic pathway, even though GI, GII, and GIII are the most studied (
Figure 2). Glyceollins are produced from the isoflavonoid branch of the phenylpropanoid pathway. Isoflavone daidzein is produced from phenylalanine and stored. Then, in the case of stress, daidzein will be metabolized into glyceollins. After multiple reduction steps, daidzein is transformed into (6aR, 11aR)-3,9 dihydroxypterocarpan. Pterocarpan is characterized by a pyrano-furano-benzene skeleton formed by the coupling of a B ring with the C ring of the isoflavonoid. Then, through the catalytic activity of CYP93A1 (also known as 3, 9 dihydroxypterocarpan 6a monooxygenase), the (6aR, 11aR)-3,9 dihydroxypterocarpan is metabolized into (-)-glycinol [
34]. From glycinol, the metabolic pathway of glyceollins differs between glyceollin I and glyceollins II and III. In both cases, the next step consists of connecting a dimethylallyl group to glycinol through the activity of a trihydroxypterocarpan dimethylallyl transferase (also known as a prenyltransferase). For GII and GIII, dimethylallyl is connected to carbon 2, which leads to the production of (6aS, 11aS)-2-dimethylallyl-3, 6a, 9-trihydroxypterocarpan (also known as glyceollidin II) [
35]. For GI, the connection of the dimethylallyl group occurs on carbon 4, which leads to the production of (6aS, 11aS)-4-dimethylallyl-3, 6a, 9-trihydroxypterocarpan (also known as glyceollidin I) [
36]. In soybeans, approximately 77 prenyltransferases have been identified. Among them, five are induced in response to
Phytophthora sojae, suggesting that they are involved in phytoalexin biosynthesis. A recent study [
37] identified GmPT01 as a prenyltransferase that localized in the plastid and presented root-specific expression. These results suggested that GmPT01 could be involved in GI biosynthesis. The final step of glyceollin biosynthesis consists of the cyclization of the dimethylallyl group by glyceollin synthase. This enzyme belongs to the family of cytochrome P450 and was identified in 1988 [
38]. To our knowledge, the specific enzymatic activity that leads to the different glyceollin isomers is not yet known.
Figure 2. Glyceollin biosynthesis pathway. The isoflavone daidzein serves as the metabolic precursor for the rapid production of glyceollins. Daidzein is produced through the isoflavonoid branch of the phenylpropanoid pathway starting from L-phenylalanine. Under stress, daidzein is transformed into (6aR, 11aR)-3,9 dihydroxypterocarpan. This molecule is metabolized by CYP93A1 into (-) glycinol. Then, a dimethylallyl group is attached to the glycinol at position 2, which leads to the production of glyceollin II and III, or at position 4, which leads to the production of glyceollin I. These two steps are catalyzed by trihydroxypterocarpan dimethylallyl transferase and glyceollin synthase, respectively.
Glyceollins are promising molecules in human health, and as such, it is important to decipher the molecular mechanism of the glyceollin biosynthesis pathway. For instance, in a recent study, the co-treatment of soybeans with silver nitrate (AgNO
3) and with the wall glucan elicitor of
Phytophthora sojae was shown to specifically enhance the production of glyceollins by not only enhancing the expression of genes encoding specific enzymes, but also by inhibiting the degradation of glyceollins [
39].
3. Metabolism and Pharmacokinetics
The beneficial antioxidant properties of isoflavones and their derivatives, phytoalexins, have generated a deep interest in their use as nutritional supplements. In terms of bioavailability and pharmacodynamics, the most studied molecule among phytoalexins is resveratrol, which has a high commercial value. However, several recent studies have focused more specifically on the metabolism and pharmacokinetics of glyceollins (
Table 1). For instance, in monkeys fed a glyceollin-enriched diet (9 mg/kg/day), the plasma level of glyceollins reached 134.2 nmol/L 4 h after oral administration and was completely undetectable 24 h later [
40]. More interestingly, while glyceollins represented approximately 50% of the isoflavones present in the diet used in the previously mentioned study, they did not represent more than 11.6% of the isoflavones found in the plasma 4 h later, due to lower glyceollin absorption or a more rapid elimination [
40].
Table 1. Bioavailability of glyceollins.
|
Animal Model
|
Treatment
|
Sample
|
Time
|
Method of Measure
|
Major Results
|
References
|
|
Female monkey
(Macaca fascicularis)
|
Diet containing glyceollin mixture 1, 134 mg/day representing 50% of total isoflavonoids in the diet
|
Plasma
|
4 h and 24 h post administration (postad)
|
Liquid chromatographic-photodiode array mass spectrometric analysis
|
Plasma concentration of glyceollins:
- 4 h: 134.2 ± 34.6 nmol/L, representing only 11.6 % of the plasma isoflavonoids level
- 24 h: Undetectable ˂ 1 nmol/L
|
Wood et al. [40]
|
|
Male ZDSD/Pco rat
|
Glyceollin mixture, gavage, 30 and 90 mg/kg
|
Plasma
|
20, 60, 120 and 240 minutes (min) postad
|
HPLC-electrospray ionization-MS/MS
|
Plama concentration of glyceollins:
- 20 min: Starts to be detectable
- the next 220 min: Remains stable
For dose 30 mg/kg: Ranges from 81.2 to 118.4 ng/mL
For dose 60 mg/kg: Ranges from 118.2 to 159.0 ng/mL
- 60 min: Peak concentration
|
Boué et al. 2012 [41]
|
|
Glyceollin mixture, gavage, 90 mg/kg/days for 2 weeks
|
Plasma, feces, and urine
|
Plasma: 3 h postad
Feces: Once daily for 2 weeks
Urine: 24 h collection postad a single dose
|
Precursor and product ion scanning using liquid chromatography coupled online with Electrospray ionization tandem mass spectrometry
|
- Rapidly absorption, glyceollins undergo phase I and phase II metabolism in the small intestine and the liver
- Metabolites of glyceollins were identified in the plasma, the urine, and the feces
Phase I conjugation: Epoxidation, hydroxylation…
Phase II conjugation: Sulfate and glucuronide conjugations…
|
Quadri et al. 2013 [43], Quadri et al. 2014 [44]
|
1 Glyceollin mixture contains glyceollin I, glyceollin II and glyceollin III.
4. Anticancer Effects
4.1. Estrogen-Dependent Effects
Like other phytoestrogens, glyceollins have a similar structure to that of the natural hormone, 17β-estradiol (E2). Therefore, their effects in estrogen-dependent diseases have been the most investigated. Breast cancer is the most frequent type of cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012 [
51]. Among the different types of breast cancer, the most common is estrogen receptor (ER)-positive cancer, which represents approximately 75% of the diagnosed cases of breast cancer [
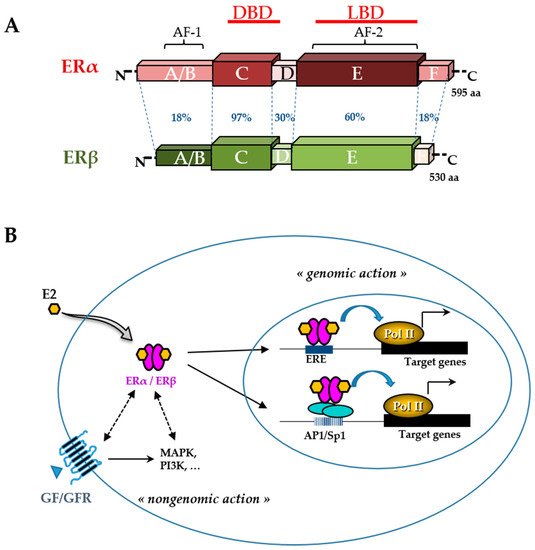
52]. The ER belongs to the nuclear receptor superfamily and is divided into two subtypes, ERα and ERβ (
Figure 3). These receptors, which function primarily as ligand-activated transcription factors, regulate various cellular functions, such as proliferation, survival, differentiation, and apoptosis [
53]. They are characterized by distinct domains comprising a conserved zinc finger DNA binding domain (DBD), a ligand binding domain (LBD), and two transactivation functions (AF). ERs typically alter the transcription of target genes by direct interaction with an estrogen-responsive element (ERE) and by recruiting transcription factors involved in chromatin remodeling [
54]. However, many E2 target genes are devoid of an ERE in their promoter. In this case, ERs can modulate their transcription by establishing protein interactions with activator protein 1 (AP1) or specificity protein 1 (Sp1) transcription factors [
55]. In addition, there are multiple levels of cross-talk between ERs and other intracellular pathways. Indeed, although ERs are mainly in the nucleus, a small amount of ERs can be present in the cytosol or near the plasma membrane. They are capable of interacting and activating various signal transduction cascades, such as mitogen-activated protein kinase (MAPK), protein kinase C, and phosphatidylinositol 3-kinase (PI3K). This type of interaction could explain the so-called ”non-genomic actions” of estrogen [
53] (
Figure 3). ERα, the major isoform in breast tissue, plays an essential role in normal mammary gland development and function as well as in breast cancer initiation and growth [
56]. ERα is bound by the natural hormone, E2, which has a pleiotropic effect and is responsible for the proliferation and survival of breast epithelial cells. Therefore, in the case of ER-positive breast cancer, ERα is a good prognostic marker and a prime target for therapy. The antagonist effect of glyceollins on ER activity was first discovered in 2001 in both HEK 293 and MCF-7 cells [
27]. More recent studies confirmed this antiproliferative effect of glyceollins in E2-dependent proliferative cell models [
25,
28]. Not only were their molecular modes of action better described, but also the glyceollin isomers were studied in isolation.
Figure 3. Estrogen receptor (ER) structure and action. The schematic structures of the two human ERα and ERβ and the percentage of homology between the different domains (annotated by the letters A to F) are indicated (A). Domains involved in DNA binding (DBD), ligand binding (LBD), ligand-independent transactivation function 1 (AF-1), and ligand-dependent transactivation function 2 (AF-2) are shown. The number of amino acids for each receptor is also indicated on the right side. Estradiol (E2) mediates numerous phenotypic effects in cells by binding to and activating ERs (B). E2 enters the cell through the lipid membranes and binds ER, which can be present in the cytoplasm and the nucleus. The activated ER forms a dimer to tightly fix chromatin directly at the estrogen-responsive element (ERE) sites or indirectly at activator protein 1 (AP1) or specificity protein 1 (Sp1) sites. ER is then able to remodel chromatin by recruiting cofactors and activating RNA polymerase II (Pol II), at target genes (genomic action). Besides, ERs can use rapid non-genomic action through the interaction with intracellular kinases (mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K),…) and the growth factor (GF) receptor (GFR) pathways.
Some phytoestrogens, such as coumestrol, daidzein, and genistein, are partial agonists of ERs. They have ER-agonist activity in low-estrogen conditions, but ER-antagonist activity in high-estrogen conditions. Unlike some phytoestrogens, the mixture of glyceollins shows only antagonist effects on ERs in low-estrogen conditions [
27,
28]. These compounds have a greater affinity for ERα than ERβ [
27], which shows their potential to treat breast cancer, where ERα is the major isoform.
GI is the most potent antiestrogenic isomer in the glyceollin mixture [
28,
57]. GI has the strongest capacity to bind to ERα due to its highest binding affinity for ERα. Examinations of the interaction between GI, GII, and GIII and the ERα ligand binding cavity in docking studies demonstrated that GI interacts with ERα in a way that is similar to tamoxifen, whereas GII and GIII can only bind to ERα in a completely different way [
28]. Among glyceollins, GI is also the strongest inhibitor of ER transcriptional activity and colony formation in MCF-7 and BG-1 cells [
28]. Its effects are different from that of its estrogenic precursor, glycinol [
58]. Among GI enantiomers, (+)-GI (synthetic enantiomer) slightly increased ERE activity, while (-)-GI (natural enantiomer) decreased the activity of both ER subtypes stimulated by E2, demonstrating potent anti-estrogenic properties [
57].
As a transcription factor, ERα either directly or indirectly regulates the expression of many genes that are involved in cell growth and proliferation. Glyceollins inhibited the expression of the E2 target genes, PgR and CXCL12 (SDF-1), in MCF-7 cells [
25,
28]. A transcriptomic assay determined that the antiproliferative effect of glyceollins in ER-positive breast cancer cells was achieved through the ER and forkhead box M1 (FOXM1) factor pathway [
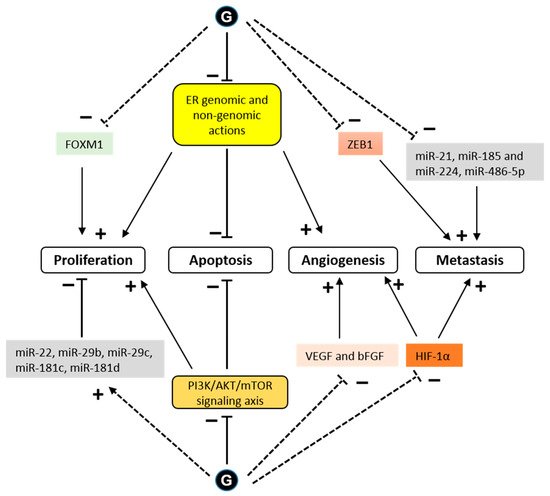
25]. Glyceollins downregulated FOXM1, which is a well-known key regulator of the cell cycle, involved in G1/S and G2/M transitions (
Figure 4).
Figure 4. Signaling pathways modulated by glyceollins in the context of cancer cells. Glyceollins (G) have been shown to directly interact with the estrogen receptor (ER), exerting antagonistic effects on ER-dependent pathways. This anti-estrogenic effect of glyceollins prevents E2-dependent proliferation and angiogenesis. In addition, glyceollins induce cell apoptosis by direct ER genomic or nongenomic (membrane-initiated) effects. The expression of forkhead box M1 (FOXM1), a key regulator of the cell cycle, is downregulated by glyceollins. Cell growth and apoptosis can also be affected by glyceollins through ER-independent pathways. Glyceollins inhibit the activity of cytoplasmic kinases, such as the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling axis. Glyceollins repress the expression of growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), and promote the expression of microRNAs (miRs) that act as tumor suppressors. Glyceollins also inhibit cell invasion and metastasis. These effects could be partially mediated by the inhibition of zinc finger E-box binding homeobox 1 (ZEB1) and hypoxia inducible factor 1 (HIF-1) expression as well as of microRNAs that enhance tumorigenesis (see text for references). Solid and dashed lines indicate direct and indirect effects, respectively. (+) indicates promoting effect and (-) indicates inhibiting effect.
Glyceollins can suppress ER transcriptional activity independently of the ligand binding domain and ERα phosphorylation. Glyceollins suppress the phosphorylation of proteins known to crosstalk with ER signaling, specifically protein S6 kinase, 70 kDa (p70S6K) [
59]. As one of the best characterized downstream targets of mTOR, p70S6K is an important regulator of cell size, protein translation, and cell proliferation [
60].
The antiestrogenic effect of glyceollins was also demonstrated in vivo. Indeed, mice xenografted with MCF-7 breast cancer cells and injected with a mixture of glyceollins showed a decrease in tumor growth [
26,
28]. Synthetic GI and GII decreased E2-dependent proliferation of mammary glands in ovariectomized mice [
25]. In female monkeys, a glyceollin-enriched diet inhibited breast proliferation stimulated by E2 [
40]. The expression of two ER-target genes in the breast epithelium, TFF1 and PgR, was markedly lower in the group with a glyceollin diet compared to that in the group treated with E2 alone.
The effect of glyceollins in other E2-dependent cell types or tissues was also investigated. GI inhibited colony formation of ovarian cancer BG-1 cells [
28]. With their antiestrogenic action, glyceollins can inhibit the growth of human ovarian cancer xenografts [
26]. After 20 days of the experiment, a treatment with a glyceollin mixture combined with E2 showed a reduction in BG-1 ovarian tumor volume (73.1%) when compared to E2 alone. These tumor-inhibiting effects corresponded to significantly lower E2-induced PgR expression in the tumors. While the use of tamoxifen or certain phytoestrogens in postmenopausal women with breast cancer is a concern because of the side effects of their estrogenic action on other tissues, such as the uterus [
61], glyceollins did not show any estrogenic effects on the uterine morphology and partially antagonized the uterotropic effects of estrogen in nude mice after 20 days of treatment [
26]. However, in our recent study, a brief treatment via the injection of GI and GII alone for 3 days showed that these compounds had no antagonistic effect on the trophic action of E2 in mouse uteri [
25]. The ability of glyceollins to function as an estrogen antagonist in the uteri of mice after a long-term treatment is a distinct advantage when compared with that of other compounds.
Furthermore, glyceollins exerted growth inhibitory effects on the human androgen-responsive prostate cancer cell line, LNCaP, by leading to G1/S arrest. Interestingly, this effect of glyceollins appeared to be mediated through the modulation of an estrogen-, but not androgen-mediated pathway [
62].
4.2. Estrogen-Independent Effect
In addition to their ER-dependent antitumor effect, glyceollins could also suppress mammary tumorigenesis through ER-independent pathways. For instance, in vitro, glyceollins decreased the proliferation of the ER-negative breast cell line, MCF10A [
25], and in vivo decreased MDA-MB-231 and MDA-MB-468 breast tumor volume in xenografted mice [
63].
One potential mechanism for metastatic spread is the epithelial to mesenchymal transition (EMT) [
64]. Characteristic of these EMT cells is a loss of E-cadherin expression and high expression of the transcription factor, ZEB1 [
65]. The ZEB1 transcription factor, known as an inducer of EMT in cancer metastasis, acts through the transcriptional repression of E-cadherin. When letrozole-resistant breast cancer cells (LTLT-Ca) were treated with GI, they exhibited morphological characteristics similar to an epithelial phenotype, and the GI treatment decreased proliferation by increasing E-cadherin and decreasing ZEB1 [
31]. These results demonstrated that GI could reverse EMT. In another study in MDA-MB-231 cells, glyceollins significantly increased miRNAs involved in EMT (miR-22, miR-29b, miR-29c, miR-30d, miR-34a, and miR-195) and tumor suppressors (miR-181c and miR-181d). There was also a significant decrease in the expression of oncomiRs promoting tumorigenesis (miR-21 and miR-193a-5p), oncomiRs promoting metastasis, such as miR-185, miR-224, and miR-486-5p, which are involved in cell migration and invasion, and miR-542-5p, which is involved in the maintenance of a mesenchymal phenotype (
Figure 4).
The formation of microvessels is a critical step in the progression of cancer [
66]. Multiple growth factors and cytokines existing in the tumor microenvironment contribute to angiogenic processes. Among them, VEGF and basic fibroblast growth factor (bFGF) are the major angiogenic factors induced by hypoxia. In vitro, glyceollins inhibited both VEGF- and bFGF-induced angiogenesis [
29]. In vivo, glyceollins strongly blocked angiogenesis in zebrafish and chick embryos as well as in mice xenografted with lung cancer cells [
29]. This effect of glyceollins could be useful not only in the case of cancers, but also for other angiogenesis diseases.
HIF-1α is a transcription factor that is constitutively expressed. Under normoxic conditions, HIF-1α is rapidly degraded by the proteins, prolyl hydroxylase domain (PHD) and factor inhibiting HIF (FIH-1) [
67]. Under hypoxic conditions, HIF-1α is stabilized and exerts its transcriptional activity. The presence of active heat shock protein 90 (HSP90) is also necessary for the rapid accumulation and activation of HIF-1α in both normoxic and hypoxic conditions [
68]. There is an association between the stability of HIF-1α and tumor growth [
69]. Lee et al. showed that glyceollins potently inhibited HIF-1α synthesis and decreased its stability by blocking the PI3K/AKT/mTOR pathway and HSP90 binding activity, respectively, under hypoxic conditions [
30]. However, surprisingly, our recent study showed that glyceollins induced HIF-1α under normoxic conditions [
25]. HIF-1α is controlled by numerous stimuli, including reactive oxygen species (ROS) [
70], whose production is activated by glyceollins at high concentrations [
71]. The effect of glyceollins on HIF-1α could be due to its role in increasing the expression of REDD1, an mTORC1 inhibitor [
25]. The inhibition of mTORC1 could be a factor involved in the antiproliferative effects of glyceollins through altering the PI3K/AKT/mTOR pathway (
Figure 4).
The anticancer effect of glyceollins may be linked to their antioxidant effect, as discussed hereafter. Pretreatment or cotreatment with glyceollins protected mice from 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis by reducing tumor formation and increasing the survival rate [
72]. This protective effect was mainly associated with their potential to induce phase 2/antioxidant enzymes that play an essential role in enhancing the hydrophilicity of exogenous carcinogens to excrete them into bile or urine. However, at high concentration, glyceollins stimulated the production of ROS, which are possibly responsible for the apoptotic activity of the compounds [
71]. Treatment with glyceollins at a high dose decreased cell viability and the mitochondrial membrane potential and increased DNA fragmentation. With their apoptotic potential, glyceollins could be exploited as antitumorigenic agents.
This entry is adapted from the peer-reviewed paper 10.3390/nu11010079