Among different thermal spraying methods, arc-spraying has been widely used due to its low operating costs and high deposition efficiency. The rapid progress of cored wire technology in arc-spraying has increased possibilities for the preparation of new Fe-based coating materials with enhanced properties by adding reinforcement particles and alloying elements to suit the different applications.
- wire arc-spraying
- Fe-based coatings
- corrosion
- wear
- high-temperature resistance
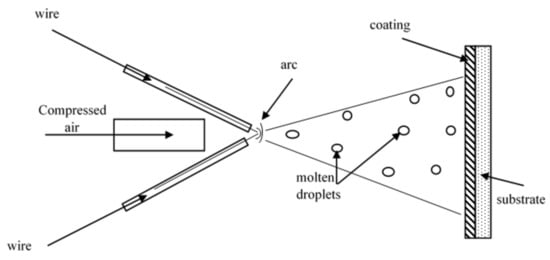
1. Working Principle of the Arc-Spraying Process
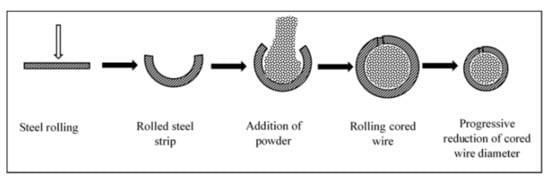
2.1. Coating Material Preparation
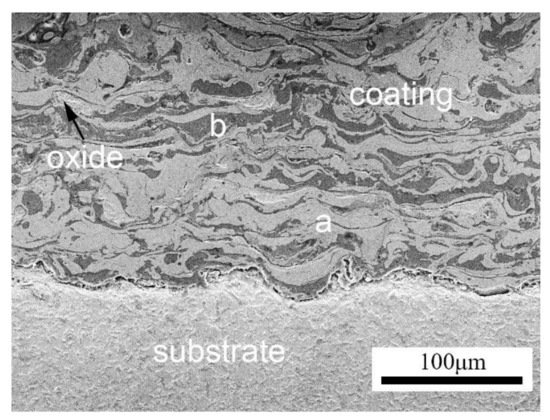
2.2. Arc-Sprayed Coating Microstructure
2. Properties of Arc-Sprayed Fe-Based Coatings
2.1. Wear Properties at Room Temperature
Table 2 summarizes the wear properties of some Fe-based coatings.
Post-treatment coating techniques such as annealing, surface remelting, and sealing improve the wear resistance of Fe-based coatings. FeNiCrAl coatings were surface-remelted by the tungsten inert gas welding. The wear resistance improved as a result of the reduced pores and cracks. The main abrasive mechanism was cutting and ploughing [70].
Transition layers or bond coat layers improve the thermal shock resistance and wear resistance by enhancing the bonding strength [71]. FeNiCrAlBRE/Ni95Al was arc-sprayed with Ni95Al applied as a transition layer to improve the adhesive strength between the coating and substrate. The coating had better wear resistance because of the low debris and shallow grooves [67]. Tian et al. [33] reported the abrasive wear properties of the 3Cr13 coating when FeNiCrAl was applied as a transition layer between the coating and the substrate. The low wear volume loss of the composite coating was due to its high hardness caused by evenly distributed Cr23C6 and (Fe, Cr) phase. The hardness-wear resistance relation could be explained by Archard’s Equation [72], as shown in Equation (1):
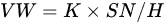
where VW is the worn volume, K is the wear coefficient, S is the sliding wear distance, N is the applied load, and H is the hardness.
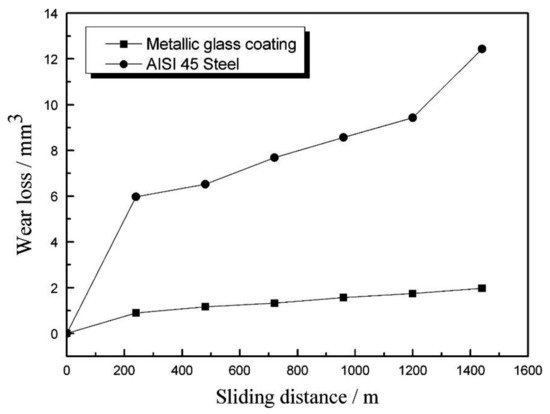
According to Equation (1), it can be stated that the high coating hardness contributes to the good wear properties. The wear rate of the coating is proportional to the applied load and sliding distance (sliding time and sliding linear speed) [73]. Volume wear losses of the AISI 45 steel substrate and the metallic glass coatings increased linearly with sliding distance (Figure 4).
3.2. Corrosion Properties at Room Temperature
Corrosion is among the leading causes of degradation of materials, especially equipment and structures exposed to marine environments. Coating defects such as pores and cracks have adverse impacts on the corrosion properties. They act as passages for the corrosive media, and they should be minimized in the coating microstructure.
The coating microstructure needs to be free of defects such as pores, oxides, unmelted particles, and cracks that accelerate the passage of electrolytes into the underlying coating. Oxides formed during the spraying process also degrade the corrosion resistance by reducing the adhesive strength between the coating splats. These defects can be minimized by optimizing the arc-spraying parameters. Crystallization of amorphous coatings during annealing causes lattice mismatch and strain, which weakens the coherence between the oxide films and metal, leading to the rupture of oxide films [81]. Thus, the annealing temperature and time should be controlled to obtain the optimum properties. Although some research has been carried out on the corrosion behavior of annealed Fe-based amorphous coatings, there is very little discussion about the corrosion behavior of annealed Fe-based crystalline alloys.
3.3. High-Temperature Properties of Arc-Sprayed Fe-Based Coatings
3.3.1. High-Temperature Oxidation Behavior
Li et al. [12] studied the oxidation properties of FeCrB(CSi) coatings with different chromium concentrations. The increase in the Cr content (17, 21, 25 at. %) improved the high-temperature oxidation resistance due to the formation of a protective Cr2O3 oxide layer that inhibited further oxygen penetration. The coating with the highest amount of Cr (25 at. %) had the least weight gain compared with the uncoated steel substrate and commercial FeCrAl coating. Boron and silicon elements controlled the oxidation of molten particles, thereby preventing Cr consumption. Li et al. [82] also investigated the influence of Cr content (15, 20, 25, 30, 35, 40 at. %) on the high-temperature oxidation properties of Fe-Cr and compared with Ni-Cr-Ti coatings to determine the suitability of Fe-based coatings as substitutes for Ni-based coatings. The Fe-Cr coatings had better high-temperature oxidation resistance than the SA213-T2 substrate used in boiler tubes. The thickness of the oxide scales and final weight gain of the Fe-Cr coatings decreased with an increase in the Cr content at 650 °C for 2 h. The Fe-35Cr and Fe-40Cr had the lowest weight gain rates, showing excellent high-temperature oxidation resistance because of the Cr2O3 and Fe2O3 oxide scales that protected the underlying substrate. The high-temperature oxidation resistance of the Fe-35Cr and Fe-40Cr coatings was close to that of the Ni-Cr-Ti coatings.
Oxidation tests conducted by Wielage et al. [8] and Vasyl et al. [85] explained the different morphology of oxides formed according to the chemical composition of individual lamellae. Figure 8 shows the oxide films that formed on different lamellae of the arc-sprayed coating [8,85]. The needle-shaped Fe2O3 formed on the lamellae with low Al and Cr content, monolithic clusters of chromium oxide or alloyed hematite (Fe, Cr)2O3 formed on the lamellae with higher Cr content, while a dense flat oxide film (Fe, Al)2O3 formed on the Al-rich lamellae. According to [86], the Fe-based coatings also formed distinctive morphologies depending on their chemical composition. At 800 °C, dense oxide films formed and with increase in temperature to 900 °C, the pores and cracks were filled by a netted oxide scale, which effectively protected the substrate during the long-term exposure.
In conclusion, the high-temperature oxidation of the Fe-based coatings depends on the formation of oxide scales that protect the coatings from further oxidation. Suitable alloying elements such as Cr and Al should be selected and their compositions optimized to enhance the high-temperature oxidation resistance.
3.3.2. High-Temperature Erosion (HTE) Behavior
High-temperature erosion (HTE) has led to the deterioration of turbines, and fluidized bed combustion boilers exposed to particulates flow and fly ash particles. Different factors such as impact angle, impingement velocity, and temperature affect erosion resistance of thermally sprayed coatings. Luo et al. [14] investigated the effects of Al content (0, 8, 15 at. %) on the erosion properties of the FeMnCrAl/Cr3C2 coating. Sample coatings with 15% Al had the best HTE resistance because of the finer microstructure with low oxide inclusions and pores. The coating without Al had the highest erosion rate under particle impacting. The high amount of oxide fractions weakened the bonding of splats and propagated cracks leading to brittle breaking and lamellar spalling. This study provides a major contribution in the advancement of knowledge on the effects of elemental composition on HTE behavior. Narrow grain size distribution of powders can be applied in arc-spraying cored wires to improve the erosion resistance as used in HVOF thermally sprayed WC-Co-Cr coatings. Narrow powder grain size distribution (36–45 µm) coating gave a higher erosion-corrosion resistance than wider grain size distribution (15–45 µm) because of the different melting behaviors. Overheating of small grains produced phases with lower erosion resistance, hence poor coating quality, while large grains were insufficiently heated, leading to a more porous coating [87]. Nanoparticle coatings produced have higher hardness and toughness as a result of the low porosity and coating defects, which can significantly affect the erosion resistance properties.
3.3.3. High-Temperature Corrosion Behavior
ot corrosion generally refers to the accelerated oxidation of metals and alloys at intermediate temperatures (600–850 °C) associated with mixed molten salts (e.g., sulphates deposited by alkali metals). Few studies on the high-temperature corrosion properties have been reported and further research on the effect of elemental compositions could be undertaken. Shukla et al. [93] fabricated arc-sprayed FeCrBSiMn coating with superior high-temperature corrosion resistance exposed to a molten salt environment (Na2SO4 − 82%Fe2(SO4)3) at 900 °C under cyclic conditions for 50 cycles. They noted that the oxides of Fe and Cr enhanced the corrosion resistance of the coatings. Xu et al. [10] prepared HVAS Fe-Al/Cr3C2 coatings on 20-grade steel. The coatings had a lower corrosion rate than the substrate. The Fe-Al matrix composites and Cr2C3 had good corrosion properties and the oxides formed protected the coatings. The corrosion rate increased with temperature but later slowed down with increased time. Cr2O3 facilitated the formation of Al2O3 that further protected the coating. Guo et al. [94] studied the hot corrosion resistance of FeCrBC in a mixed solution of Na2SO4 + K2SO4 (7:3) at 700 °C for 156 h. Thin films of Cr2O3 formed on the coating and separated the alloy from the mixed salt solution, thus providing better resistance to hot corrosion. In a comparison study by Li et al. [11], the arc-sprayed FeCrSiB coatings provided better hot corrosion resistance than FeCr, approaching that of NiCrTi coating. The weight gain for the hot corrosion has been calculated as follows:
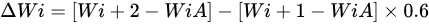
where, Wi is the mass of the sample before the ith corrosion, Wi+1 is the mass after the first salt coating, Wi+2 is the mass after the ith corrosion, A is the total surface area of the sample, and 0.6 is the coefficient for deducting the salt film crystal water.
3.3.4. High-Temperature Wear Behavior
Several studies have shown the relation between the coating integrity and the coating’s high-temperature wear performance. Stress concentration occurs at the defects such as cracks on the coating, which propagate coating failure during the wear process [98]. The coating hardness, cohesive strength of the coating, and bond strength of the coating with substrate influence the high-temperature wear properties.
The phase composition of the coatings and the oxides formation controlled the high-temperature wear, oxidation, corrosion, and erosion resistance. The oxide scales generated stresses that determined the spalling and cracking of the coatings. Adding alloying elements such as Al and Cr is vital to form oxides that reduce the wear and corrosion rate at high temperatures. The coating defects undermine the coating protection at high temperatures, hence the need to minimize them through process optimization. The high-temperature wear resistance has mostly been characterized by the weight gain and weight reduction of the coatings. The degradation of coatings in corrosive medium at high temperature involves the combination of wear process and the electrochemical corrosion process. The corrosion-wear behavior of arc-sprayed Fe-based coatings should be investigated at high temperatures to further understand the tribological properties.
4. Conclusions and Future Scope Recommendations
-
The density, size, and structure of feedstock powders influence the phase composition of the deposited coatings in HVOF and APS thermal spraying methods. Cored wires in arc-spraying can explore the use of different-sized powders as filling materials to optimize the coating properties. Coating powders of arc-sprayed cored wires can apply nanoscale particles that result in densely packed nanostructured coatings [57]. Arc-sprayed FePSiNb coatings exhibited a nanoscale structure with a grain size range from 12 to 50 nm with good wear resistance properties [63]. More work will need to be done to determine the production and study of properties of arc-sprayed nanostructured coatings.
-
The spraying parameters play an important role in determining the microstructural properties of the coatings. Optimizing methods such as response surface methodology (RSM) analyzes the interaction between spray parameters and their influence on the coating properties. The effects of process parameters on the amorphization of the arc-sprayed coatings could be studied to maximize the amorphous content of Fe-based amorphous coatings.
-
The arc-sprayed Fe-based coatings have better hardness and wear resistance properties than conventional alloys due to the dense microstructure, the dispersion strengthening of the amorphous/nanocrystalline phases, and reinforcement ceramic particles. The elastic properties also determine the wear resistance of the Fe-based coatings.
-
To increase the corrosion resistance, the coating defects (oxides, pores, and cracks) in the Fe-based coatings should be minimized by optimizing spray parameters to prevent the deterioration of coating properties in corrosive media.
-
The high-temperature properties of the Fe-based coating are mainly affected by the microstructure and the elemental composition. The reinforcement ceramic particles added to the Fe-based alloys improve the tribological and high-temperature coating performance while the amorphous phase content is characterized by fewer dislocations, microcracks, and grain boundaries enhancing the properties of the Fe-based amorphous coatings. Future research should focus on understanding the combined corrosion-wear behavior of arc-sprayed Fe-based coatings at elevated temperatures.
-
Adding appropriate alloying elements such as Al and Cr to Fe-based coatings improves high-temperature protection by forming oxide scales that prevent further oxidation of the underlying substrate. Future research should investigate the influence of different elements on high-temperature properties of Fe-based coatings.
This entry is adapted from the peer-reviewed paper 10.3390/nano11102527
