Drimia (synonym Urginea) plants are bulbous plants belonging to the family Asparagaceae (formerly the family Hyacinthaceae) and are distinctive, powerful medicinal plants. Just some species are indigenous to South Africa and have been traditionally utilized for centuries to cure various diseases and/or ailments. They have been recognized among the most famous and used medicinal plants in South Africa. Traditionally, the plants are used for various illnesses such as dropsy, respiratory disease, bone and joint complications, skin disorders, epilepsy and cancer. A number of studies have reported biological properties such as antiviral, antibacterial, antioxidant and anti-inflammatory, immunomodulatory, and anticancer activities. Their bulbs are a popular treatment for colds, measles, pneumonia, coughs, fever and headaches.
- Drimia
- Urginea
- poisonous
- compounds
- toxicity
- bulbous plants
1. Introduction
2. Botanical Characteristics and Geographical Distribution of Drimia
3. Biological Activities of Drimia spp.
3.1. Antimicrobial Activity
3.2. Anti-Inflammatory Activity
3.3. Antioxidant Activity
3.4. Anticancer Activity
| Drimia Species | Biological Activity | Part Used | Extraction Form | Chemical Composition | Ref. |
|---|---|---|---|---|---|
| D. sanguinea | Antibacterial activity, antifungal, antioxidant, anticytotoxicity | Bulbs | Methanol extract, petroleum ether, extract | Pentanoic acid, n-hexadecanoic acid, 1-nonadecene, hexadecanoic acid, ethyl ester, di-isooctyl phthalate, α-sitosterol | [65] |
| D. indica | Antifungal activity | Bulbs | Crude extract, methanol extract | O-glycosyl flavanone, O-glycosyl flavone and C-glycosyl flavone |
[32] |
| D. indica | Anthelmintic activity | Bulbs | Aqueous extract, crude extract | Not specified | [32] |
| D. indica | Antitumor activity | Bulbs | Crude extract | a C-glycosyl flavone (5,7-dihydroxy-2-[40-hydroxy-30-(methoxymethyl) phenyl]-6-C-βglucopyranosyl flavone | [66][67] |
| D. indica | Antibacterial activity | Bulbs | Aqueous extract, ethanol extract, methanol extract | Not specified | [68][69][70] |
| D. indica | Antioxidant activity | Bulbs | Methanol extract, chloroform extract | Flavonoids, phenolic and proanthocyanidins | [71][72][73] |
| D. coromandeliana, D. govindappae, D. indica, D. nagarjunae, D. polyantha, D. raogibikei and D. razii | Antioxidant activity | Bulbs | Hydrochloric acid extract and methanol extract | Total phenolics and proanthocyanidins | [57] |
| D. indica | Antidiabetic activity | Bulbs | Ethanol extract | Not specified | [74] |
| D. indica | Anti-inflammatory | Bulbs | Alcoholic extract | Not specified | [75] |
| D. robusta | Antibacterial, anti-inflammatory, antihypertensive and anticancer activities | Leaf Bulb | Alcoholic extract | Cardiac glycosides, bufadienolides | [76] |
| D. maritime | Antibacterial, anti-inflammatory and anticancer activities | Bulbs | Ethanol extract | Cardiac glycosides, bufadienolides sclerosis and triterpenoids. | [77] |
| D. robusta | Antibacterial and anticandidal activities | Bulbs and leaves | Petroleum ether, dichloromethane, ethanol and water extracts | Phenolic compounds | [78] |
| D. maritima | Antimalarial activity and cytotoxicity | Bulb | Aqueous extract | Not specified | [79] |
| D. maritima | Asthma effect | Bulb | Squill oxymel (a traditional form of Drimia maritima), simple oxymel | Not specified | [80] |
| D. maritima | Anticancer effects | Whole plant | Methanol extract | Cardiac glycoside | [81] |
| D. maritima | Analgesic effects | Squill bulb | Proscillaridin A, taxifolin and scilliroside | Not specified | [82] |
| D. maritima | Antioxidant activity and antihemolytic effect | Flowers | Ethanolic, chloroform and ethyl acetate extract | Total phenolic, flavonoid and tannin | [57] |
| D. robusta | Antibacterial activity | Bulb | Ethanolic extract | Cardiac glycosides (2-deoxy sugars), bufadienolides | [83] |
| D. maritima | Antioxidant activities | Leaves and tubers. | Ethanol, methanol, acetone extracts | Phenolic compounds | [57] |
| D. macrocentra and U. riparia | Anticancer activity | Bulbs | Extracts | Bufadienolides, rubellin and riparianin | [84] |
| D. maritima | Acaricidal activity | Leaves and bulbs | Methanol, ethanol, acetone and butanol | Bufadienolides derivatives | [85] |
| D. numidica | Antioxidant activity | Flowers, scales, leaves, bulbs and roots | Methanolic | Bufadienolides and total phenolic content | [86] |
| D. nagarjunae | Anticancer activity | Bulbs and leaves | Ethyl acetate and chloroform | Acetic acid, (D,L)-malic acid, hexadecanoic acid, ethyl[4-t-Butyl-2,6-bis(1-methoxy-1-methylethyl)phenyl]phosphinate, octadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | [51] |
| D. maritima | Decreasing dyspareunia and increasing sexual satisfaction | Squill oil | N/A | Flavonoids | [87] |
| D. maritima | Acaricidal activity | Leaves and bulbs | Methanol, ethanol, acetone and butanol | Bufadienolides | [88] |
4. Phytochemicals of Drimia spp.
4.1. Cardiac Glycosides
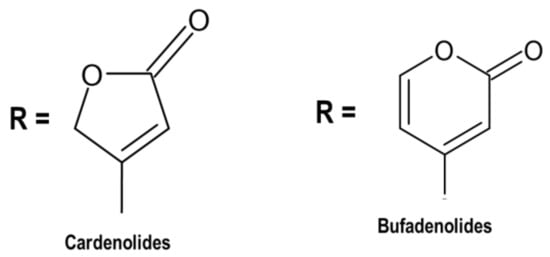
4.2. Phenolic Compounds
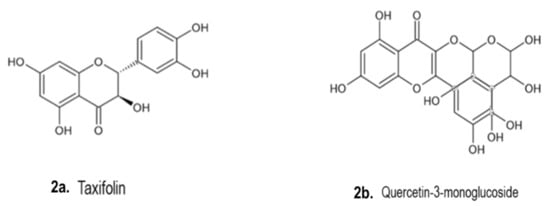
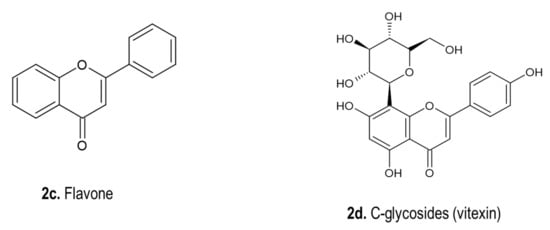
4.3. Phytosterols
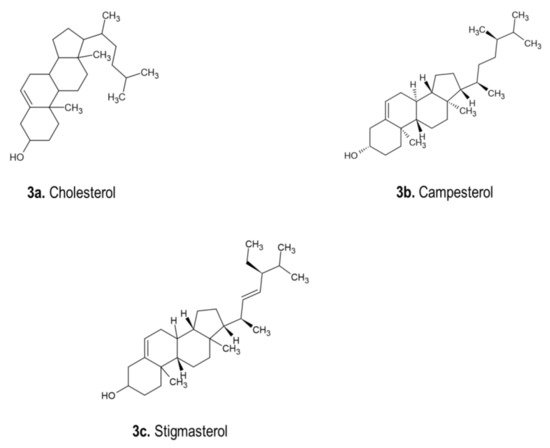
4.4. Miscellaneous Compounds
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13091385
References
- Bozorgi, M.; Amin, G.; Shekarchi, M.; Rahimi, R. Traditional medical uses of Drimia species in terms of phytochemistry, pharmacology and toxicology. J. Tradit. Chin. Med. 2017, 37, 124–139.
- Marx, J.; Pretorius, E.; Espag, W.J.; Bester, M.J. Urginea sanguinea: Medicinal wonder or death in disguise? Environ. Toxicol. Pharmacol. 2005, 20, 26–34.
- Crespo, M.B.; Martínez-Azorín, M.; Alonso, M.Á. The identity of Drimia purpurascens, with a new nomenclatural and taxonomic approach to the “Drimia undata” group (Hyacinthaceae = Asparagaceae subfam. Scilloideae). Plant Syst. Evol. 2020, 306, 1–18.
- Rasethe, M.T. The Utilization and Management of Selected Listed-Threatened or Protected Species in the Limpopo Province, South Africa. Master’s Thesis, University of Limpopo, Limpopo, South Africa, 2017.
- Ndhlala, A.R.; Ncube, B.; Okem, A.; Mulaudzi, R.B.; Van Staden, J. Toxicology of some important medicinal plants in southern Africa. Food Chem. Toxicol. 2013, 62, 609–621.
- Watt, J.M.; Breyer-Brandwijk, M.G. The Midicinal and Poisonous Plants of Southern and Easthern Africa, 1962, no. 581.96 W38. Available online: http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=UACHBC.xis&method=post&formato=2&cantidad=1&expresion=mfn=058220 (accessed on 20 April 2021).
- Hutchings, A. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996; p. 464. ISBN 0869808931.
- Van Wyk, B.E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000.
- Foukaridis, G.N.; Osuch, E.; Mathibe, L.; Tsipa, P. The ethnopharmacology and toxicology of Urginea sanguinea in the Pretoria area. J. Ethnopharmacol. 1995, 49, 77–79.
- Moll, E.J.; Strebel, R.C. Poisonous Plants; Struik Publishers: Cape Town, South Africa, 1989.
- Van Der Bijl, P. Cardiotoxicity of plants in South Africa. Cardiovasc. J. Afr. 2012, 23, 476.
- Pieter, V.; Pieter, V. Into small pieces, making botanical identification difficult or impossible. Plants known to be toxic contain chemical constituents that can affect a wide range of organ systems; these have been documented in a number of publications. As far as the cardiovascular system. Cardiovasc. J. Afr. 2012, 23, 9.
- Van Vuuren, S.; Williams, V.L.; Sooka, A.; Burger, A.; Van Der Haar, L. Microbial contamination of traditional medicinal plants sold at the Faraday muthi market, Johannesburg, South Africa. S. Afr. J. Bot. 2014, 94, 95–100.
- Moichwanetse, B.I.; Ndhlovu, P.T.; Sedupane, G.; Aremu, A.O. Ethno-veterinary plants used for the treatment of retained placenta and associated diseases in cattle among Dinokana communities, North West Province, South Africa. S. Afr. J. Bot. 2020, 132, 108–116.
- Martinez-Azorin, M.; Crespo, M.B.; Dold, A.P. Trimelopter craibii (Hyacinthaceae, Ornithogaloideae), a new species from the North West Province of South Africa. Phytotaxa 2013, 87, 50–60.
- Lekhak, M.M.; Yadav, P.B.; Yadav, S.R. Cytogenetic Studies in Indian Drimia Jacq. (Urgineoideae: Hyacinthaceae). In Chromosome Structure and Aberrations; Springer: New Delhi, India, 2017; pp. 141–165.
- De Wet, B. Medicinal plants and human health. S. Afr. Pharm. J. 2011, 78, 38–40.
- Naudé, T.W. The Occurence and Significance of South African Cardiac Glycosides. J. S. Afr. Biol. Soc. 1977, 18, 7–20.
- Kellerman, T.S.; Coetzer, J.A.W.; Naudé, T.W.; Botha, C.J. Plant Poisonings and Mycotoxicoses of Livestock in Southern Africa, 2nd ed.; Oxford University Press Southern Africa: Cape Town, South Africa, 2005.
- Majinda, R.R.; Waigh, R.D.; Waterman, P.G. Bufadienolides and other constituents of Urginea sanguinea. Planta Med. 1997, 63, 188–190.
- Yadav, P.B.; Lekhak, U.M.; Ghane, S.G.; Lekhak, M.M. Phytochemicals, antioxidants, estimation of cardiac glycoside (Scillaren A) and detection of major metabolites using LC-MS from Drimia species. S. Afr. J. Bot. 2020.
- “Drimia maritima“. World Checklist of Selected Plant Families. Royal Botanic Gardens, Kew. Available online: https://wcsp.science.kew.org/namedetail.do?name_id=305015 (accessed on 23 April 2021).
- Jacquin, N.J. “Drimia elata”. Collectaneorum Supplementum; Wappler: Vienna, Austria, 1797; pp. 38–39.
- Evans Pole, I.B. The Flowering Plants of South Africa. Nature 1921, 107, 40.
- Raimondo, D.; von Staden, L.; Foden, W.; Victor, J.E.; Helme, N.A.; Turner, R.C.; Kamundi, D.A.; Manyama, P.A. Red List of South African Plants; South African National Biodiversity Institute: Pretoria, South Africa, 2009.
- Manning, J.; Deacon, J.; Goldblatt, P. A review of the Schizobasis group of Drimia Jacq. (Hyacinthaceae: Urgineoideae), and the new species D. sigmoidea from Western Cape, South Africa. S. Afr. J. Bot. 2014, 94, 263–269.
- Baskaran, P.; Singh, S.; Van Staden, J. In vitro propagation, proscillaridin A production and antibacterial activity in Drimia robusta. Plant Cell Tissue Organ Cult (PCTOC) 2013, 114, 259–267.
- Wang, L.; Amin, A.K.; Khanna, P.; Aali, A.; McGregor, A.; Bassett, P.; Gopal Rao, G. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021, 76, 796–803.
- Tshitshi, L.; Manganyi, M.C.; Montso, P.K.; Mbewe, M.; Ateba, C.N. Extended Spectrum Beta-Lactamase-Resistant Determinants among Carbapenem-Resistant Enterobacteriaceae from Beef Cattle in the North West Province, South Africa: A Critical Assessment of Their Possible Public Health Implications. Antibiotics 2020, 9, 820.
- Kaptchouang Tchatchouang, C.D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis outbreak in South Africa: A comparative analysis with previously reported cases worldwide. Microorganisms 2020, 8, 135.
- Nair, R.; Shah, A.; Baluja, S.; Chanda, S. Synthesis and antibacterial activity of some Schiff base complexes. J. Serbian Chem. Soc. 2006, 71, 733–744.
- Chittoor, M.S.; Binny, A.R.; Yadlapalli, S.K.; Cheruku, A.; Dandu, C.; Nimmanapalli, Y. Anthelmintic and antimicrobial studies of Drimia indica (Roxb.) Jessop. bulb aqueous extracts. J. Pharm. Res. 2012, 5, 3677–3686.
- Pandey, D.; Gupta, A.K. Antimicrobial activity and phytochemical analysis of Urginea indica from Bastar district of Chhattisgarh. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 273–281.
- Maazoun, A.M.; Hamdane, A.M.; Belhadj, F.; Marzouki, M.N. In vitro antimicrobial activity of Urginea maritima (L.) Baker bulb extract against food-borne pathogens. J. Mater. Environ. Sci. 2019, 10, 1053–1061.
- Baskaran, P.; Kumari, A.; Van Staden, J. Analysis of the effect of plant growth regulators and organic elicitors on antibacterial activity of Eucomis autumnalis and Drimia robusta ex vitro-grown biomass. Plant Growth Regul. 2018, 85, 143–151.
- Pandey, D.; Gupta, A.K. Bioactive Compound in Urginea indica (Kunth.) from Bastar and its Spectral Analysis by HPLC, UV-Vis, FT-IR, NMR, and ESI-MS. SN Compr. Clin. Med. 2019, 1, 241–254.
- Shenoy, S.R.; Kameshwari, M.S.; Swaminathan, S.; Gupta, M.N. Major antifungal activity from the bulbs of Indian squill Urginea indica is a chitinase. Biotechnol. Prog. 2006, 22, 631–637.
- Matotoka, M.M.; Ndhlala, A.R.; Masoko, P. In vitro inhibition of HIV-1 reverse transcriptase and anti-inflammatory activities of some herbal concoctions sold in the Limpopo Province. S. Afr. J. Bot. 2019, 126, 65–69.
- Semenya, S.S.; Potgieter, M.J.; Erasmus, L.J.C. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: Their distribution, harvesting, conservation and threats. S. Afr. J. Bot. 2013, 87, 66–75.
- Kamano, Y.; Satoh, N.; Nakayoshi, H.; Pettit, G.R.; Smith, C.R. Rhinovirus Inhibition by Bufadienolidesl. Chem. Pharm. Bull. 1988, 36, 326–332.
- Prabakaran, R.; Joseph, B.; Pradeep, P.N. Phyto medicinal compounds from Urginea indica Kunth: A synthetic drugs potential alternative. J. Pharm. Res. Int. 2016, 1–9.
- Belhaddad, O.E.; Charef, N.; Amamra, S.; Zerargui, F.; Baghiani, A.; Khennouf, S.; Arrar, L. Chromatographic fractionation, antioxidant and antibacterial activities of Urginea maritima methanolic extract. Pak. J. Pharm. Sci. 2018, 30, 127–134.
- Sato, N.; Muro, T. Antiviral activity of scillarenin, a plant bufadienolide. Jpn. J. Microbiol. 1974, 18, 441–448.
- Raj, M.S.; Kameshwari, M.S. Extraction, isolation and identification of bioactive compounds in Urginea indica. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 24–29.
- Shukla, R.; Kumar, A.; Prasad, C.S.; Srivastava, B.; Dubey, N.K. Antimycotic and antiaflatoxigenic potency of Adenocalymma alliaceum Miers. on fungi causing biodeterioration of food commodities and raw herbal drugs. Int. Biodeterior. Biodegrad. 2008, 62, 348–351.
- Scogin, R. Anthocyanins of the Bignoniaceae. Biochem. Syst. Ecol. 1980, 8, 273–276.
- Otimenyin, S.O. Antiinflammatory Medicinal Plants: A Remedy for Most Disease Conditions? In Natural Products and Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 411–431.
- Witbooi, H.; Okem, A.; Makunga, N.P.; Kambizi, L. Micropropagation and secondary metabolites in Agathosma betulina (Berg.). S. Afr. J. Bot. 2017, 111, 283–290.
- Bakasatae, N.; Kunworarath, N.; Yupanqui, C.T.; Voravuthikunchai, S.P.; Joycharat, N. Bioactive components, antioxidant, and anti-inflammatory activities of the wood of Albizia myriophylla. Braz. J. Pharmacogn. 2018, 28, 444–450.
- Kazemi Rad, H.; Memarzia, A.; Amin, F.; Boskabady, M.H. Relaxant Effect of Urginea maritima on Tracheal Smooth Muscle Mediated by the Effect on Beta-2 Adrenergic, Muscarinic Receptors and Calcium and Potassium Channels. Evid Based Complement. Alternat. Med. 2021, 2021, 1–9.
- Alluri, N.; Majumdar, M. In vitro anti-cancer potential and GC-MS analysis of Drimia nagarjunae, an endangered medicinal plant. Bangladesh J. Pharmacol. 2015, 10, 303–307.
- Fürst, R.; Zündorf, I.; Dingermann, T. New knowledge about old drugs: The anti-inflammatory properties of cardiac glycosides. Planta Med. 2017, 83, 977–984.
- Akhtar, G.; Shabbir, A. Urginea indica attenuated rheumatoid arthritis and inflammatory paw edema in diverse animal models of acute and chronic inflammation. J. Ethnopharmacol. 2019, 238, 111864.
- Tahri, Y.; Koubaa, I.; Frikha, D.; Maalej, S.; Allouche, N. Chemical Investigation and Biological Valorization of Two Essential Oils Newly Extracted from Different Parts of Drimia maritima. J. Essent. Oil Bear Plants. 2020, 23, 1022–1034.
- Mammadov, R.; Makasçı-Afacan, A.; Uysal-Demir, D.; Görk, Ç. Determination of antioxidant activities of different Urginea maritima (L.) Baker plant extracts. Iran. J. Chem Chem Eng (IJCCE) 2010, 29, 47–53.
- Mahato, D.; Sahu, A.P.; Sharma, H.P. Phytochemical and antioxidant evaluation of Urginea indica Kunth. Indian J. Tradit Knowdl. 2018, 17, 783–788.
- Rajput, B.; Golave, A.; Yadav, S.; Jadhav, J.P. Total phenolic concentrations and antioxidant activities in Drimia sp. J. Herbs Spices Med. Plants 2018, 24, 28–36.
- Rezzagui, A.; Senator, A.; Benbrinis, S.; Bouriche, H. Free Radical Scavenging Activity, Reducing Power and Anti-Hemolytic Capacity of Algerian Drimia maritima Baker Flower Extracts. J. Drug Deliv. Ther. 2020, 10, 70–78.
- Nyambe, M.N.; Beukes, D.R.; Van De Venter, M.; Swanepoel, B.; Hlangothi, B.G. Isolation and characterisation of altissimin: A novel cytotoxic flavonoid C-apioglucoside from Drimia altissima (Asparagaceae). Nat. Prod. Res. 2021, 35, 717–725.
- Langat, L.; Langat, M.K.; Wetschnig, W.; Knirsch, W.; Mulholland, D.A. Antiproliferative Bufadienolides from the Bulbs of Drimia altissima. J. Nat. Prod. 2021, 84, 608–661.
- Babu Bevara, G.; Naveen Kumar, A.D.; Koteswramma, K.L.; Kumar, B.A.; Kumari, S.; Sastry Yarla, N.; Malla, R.R. C-glycosyl flavone from Urginea indica inhibits growth and dissemination of ehrlich ascites carcinoma cells in mice. Anti Cancer Agent Med. Chem. 2017, 17, 1256–1266.
- Winnicka, K.; Bielawski, K.; Bielawska, A.; Miltyk, W. Apoptosis-mediated cytotoxicity of ouabain, digoxin and proscillaridin A in the estrogen independent MDA-MB-231 breast cancer cells. Arch. Pharm. Res. 2007, 30, 1216–1224.
- Fouché, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharm. 2008, 119, 455–461.
- Saket, K.; Salari, R.; Saburi, E.; Yousefi, M.; Khodadoust, M.A.; Hadi, M.; Afshari, J.T. Anti-cancer effect of Urginea maritima bulb extract invitro through cell cycle arrest and induction of apoptosis in human breast cancer cell lines. Curr. Drug Discov. Technol. 2020.
- Asong, J.A.; Amoo, S.O.; Mcgaw, L.J.; Nkadimeng, S.M.; Aremu, A.O.; Otang-mbeng, W.J.P. Antimicrobial activity, antioxidant potential, cytotoxicity and phytochemical profiling of four plants locally used against skin diseases. Plants 2019, 8, 350.
- Hossain, M.S.; Khalequeuzzaman, M.; Hasan, M.N.; Islam, M.A.F.; Rana, M.S. Evaluation of Anticancer Potential Of The Bulbs of Urginea Indica. British J. Med. Health Sci. (BJMHS) 2020, 2, 117–121.
- Dhar, M.L.; Dhar, M.M.; Dhawan, B.N.; Mehrotra, B.N.; Ray, C. Screening of Indian plants for biological activity: I. Indian J. Exp. Biol. 1968, 6, 232–247.
- Khare, C.P. Indian Herbal Remedies; Springer: Berlin, Germany, 2004; pp. 464–465.
- Deepak, A.V.; Salimath, B.P. Antiangiogenic and proapoptotic activity of a novel glycoprotein from U. indica is mediated by NF-kB and Caspase activated DNase in ascites tumor model. Biochimie 2006, 88, 297–307.
- Cáceres, A.; Menéndez, H.; Méndez, E.; Cohobón, E.; Samayoa, B.E.; Jauregui, E.; Peralta, E.; Carrillo, G. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J. Ethnopharmacol. 1995, 48, 85–88.
- Shokeen, P.; Bala, M.; Tandon, V. Evaluation of the activity of 16 medicinal plants against Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 2009, 33, 86–91.
- Thatoi, H.N.; Panda, S.K.; Rath, S.K.; Dutta, S.K. Antimicrobial activity and ethnomedicinal uses of some medicinal plants from Similipal Biosphere Reserve, Orissa. Asian J. Plant. Sci. 2008, 7, 260–267.
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm Rev. 2010, 4, 118–126.
- Soni, L.K.; Jain, S.K.; Dobhal, S.; Parasher, P.; Dobhal, M.P. Free radical scavenging activity of Urginea indica, Alhagi maurorum, Crinum asiaticum and Prosopis cineraria. Int. J. Pharm. Phytochem. Res. 2015, 7, 311–314.
- Gupta, A.; Singh, S.K.; Yadav, A.K. Pharmacological evaluation of antidiabetic activity of Urginea indica in laboratory animals. Int. J. Nutr. Pharm. Neurol. Dis. 2015, 5, 63–68.
- Rahman, M.M.; Chowdhury, J.A.; Habib, R.; Saha, B.K.; Salauddin, A.D.M.; Islam, M.K. Anti-inflammatory, anti-arthritic and analgesic activity of the alcoholic extract of the plant Urginea indica kunth. Int. J. Pharm. Sci. Res. 2011, 2, 2320–2324.
- Ngugi, G.W.; Jager, A.K.; Van Staden, J. In vitro propagation of Drimia robusta. Bak. S. Afr. J. Bot. 1998, 64, 266–268.
- Hammouda, F.M.; Ismail, S.I.; Abdel-Azim, N.S.; Shams, K.A. Urginea maritima (L.) Baker Liliaceae. Baker. J. Linn. London (Bot.) 1873, 13, 221.
- Ncube, B.; Finnie, J.F.; Van Staden, J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. S. Afr. J. Bot. 2011, 77, 387–396.
- Sathiyamoorthy, P.; Lugasi-Evgi, H.; Schlesinger, P.; Kedar, I.; Gopas, J.; Pollack, Y.; Golan-Goldhirsh, A. Screening for cytotoxic and antimalarial activities in desert plants of the negev and bedouin market plant products. Pharm. Biol. 1999, 37, 188–195.
- Nejatbakhsh, F.; Karegar-Borzi, H.; Amin, G.; Eslaminejad, A.; Hosseini, M.; Bozorgi, M.; Gharabaghi, M.A. Squill Oxymel, a traditional formulation from Drimia maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: A pilot, triple-blind, randomized clinical trial. J. Ethnopharmacol. 2017, 196, 186–192.
- Bayazıt, V.; Konar, V. Analgesic effects of scilliroside, proscillaridin-a and taxifolin from squill bulb (Urginea maritima) on pains. Digest. J. Nanomater. Biostruct. 2010, 5, 465–467.
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Goransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757.
- Luyt, R.P.; Jäger, A.K.; Van Staden, J. The rational usage of Drimia robusta Bak. in traditional medicine. S. Afr. J. Bot. 1999, 65, 291–294.
- Moodley, N.; Crouch, N.R.; Mulholland, D.A. Bufadienolides from Drimia macrocentra and Urginea riparia (Hyacinthaceae: Urgineoideae). Phytochemistry 2007, 68, 2415–2419.
- Rhimi, W.; Camarda, A.; Saidi, M.; Boulila, A.; Otranto, D.; Cafarchia, C. Chemical characterization and acaricidal activity of Drimia maritima (L) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019, 268, 61–66.
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150.
- Karimi, F.; Babazadeh, R.; Jouya, S.; Zojaji, A. Squill oil for decreasing dyspareunia and increasing sexual satisfaction in menopausal women: A triple-blind randomized controlled trial. Avicenna J. Phytomed. 2021, 11, 464–472.
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Metabolic fate of cardiac glycosides and flavonoids upon fermentation of aqueous sea squill (Drimia maritima L.) extracts. J. Pharm. Biomed. Anal. 2015, 110, 100–109.
- Sharma, H.J.; Devi, N.S. Phytochemical Analysis of Drimia Species. Int J. Appl. Sci. Res. Rev. 2017, 4, 12.
- Stoll, A.; Suter, E.; Kreis, W.; Bussemaker, B.; Hofmann, A. Die herzaktiven substanzen der meerzwiebel. Scillaren A Helv. Chim. Acta 1933, 16, 703–733.
- Bartnik, M.; Facey, P.C. Glycosides. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 101–161.
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Dis. 2008, 7, 926–935.
- Patel, S. Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. 2016, 84, 1036–1041.
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Maldonado-Navas, D.; García-Domínguez, I.; López-Lázaro, M. Evaluating the cancer therapeutic potential of cardiac glycosides. Nat. Bioact. Cancer Treat. Prev. 2014, 2014, 794930.
- Mijatovic, T.; Dufrasne, F.; Kiss, R. Cardiotonic steroids-mediated targeting of the Na+/K+-ATPase to combat chemoresistant cancers. Curr. Med. Chem. 2012, 19, 627–646.
- Challinor, V.L.; De Voss, J.J. Open-chain steroidal glycosides, a diverse class of plant saponins. Nat. Prod. Rep. 2013, 30, 429–454.
- Morsy, N. Cardiac glycosides in medicinal plants. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H., Ed.; IntechOpen: London, UK, 2017.
- He, Y.; Khan, M.; Yang, J.; Yao, M.; Yu, S.; Gao, H. Proscillaridin A induces apoptosis, inhibits STAT3 activation and augments doxorubicin toxicity in prostate cancer cells. Inter. J. Med. Sci. 2018, 15, 832.
- Iizuka, M.; Warashina, T.; Noro, T. Bufadienolides and a new lignan from the bulbs of Urginea maritima. Chem. Pharm. Bull. 2001, 49, 282–286.
- Karaś, K.; Sałkowska, A.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Cardiac glycosides with target at direct and indirect interactions with nuclear receptors. Biomed. Pharmacother. 2020, 127, 110106.
- Liang, X.M.; Jin, Y.; Wang, Y.P.; Jin, G.W.; Fu, Q.; Xiao, Y.S. Qualitative and quantitative analysis in quality control of traditional Chinese medicines. J. Chromatogr. A 2009, 1216, 2033–2044.
- Knittel, D.N.; Kammerer, D.R.; Stintzing, F.C. Characterization of phenolic compounds and cardiac glycosides from red sea squill (Urginea maritima (L.) Baker) by RP-HPLC-DAD-MSn. Planta Med. 2013, 79, 13.
- Sultana, N.; Akter, K.; Nahar, N.; Khan, M.S.H.; Mosihuzzaman, M.; Sohrab, M.H.; Krohn, K. Novel flavonoid glycosides from the bulbs of Urginea indica Kunth. Nat. Prod. Res. 2010, 24, 1018–1026.
- Rishi, A.; Sneha, S. Quantitative estimation of-sitosterol and stigmasterol in Gloriosa superba L. and Urginea indica (Roxb.) Kunth. J. Med. Plants Res. 2013, 7, 3127–3130.
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and recovery methods. Biores. Technol. 2007, 98, 2335–2350.
- Mulholland, D.A.; Schwikkard, S.L.; Crouch, N.R. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod Rep. 2013, 30, 1165–1210.
- Kim, H.M.; Lee, J.S.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.H.; Jang, D.S. A new canthinone-type alkaloid isolated from Ailanthus altissima Swingle. Molecules 2016, 21, 642.
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909.
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herb. Med. Pharmacol. 2018, 7, 1–7.
