In vitro culture of endothelial cells to form capillary-like networks is essential in tissue engineering. Vascular endothelial growth factor (VEGF) is one of the primary signal proteins stimulating blood vessel formation. This growth factor can be soluble in the medium or protein-bound to the substrate. However, less attention has been paid to distinguishing the specific stimulations by soluble and bound VEGF. We conducted a series of experiments to explore the respective effects of these two VEGF forms. An in-house synthesized biogel comprising a definite concentration of collagen and fibronectin was designed to cultivate human umbilical vein endothelial cells to form the capillary-like network. Collagen served as the primary substrate for cell attachment. Fibronectin provided the surface to bind soluble VEGF in the culture medium to create the bound VEGF. The experiment of adding VEGF-blocking-peptide was conducted to prevent the formation of VEGF bound to the fibronectin domains, to distinguish the respective effects of the soluble and bound VEGF. With the in-house biogel of definite components, we were able to clarify the different roles of soluble and bound VEGF. The results indicated that the soluble VEGF promptly induced the cells to change from round to elongated shape, which contributed to forming network cords. Simultaneously, the bound VEGF provided long-term stimulation, causing the cells to migrate and differentiate into the final capillary-like network.
- capillary-like network
- endothelial cells
- morphology
- vascularization
- VEGF
1. Introduction
2. Analysis on Results
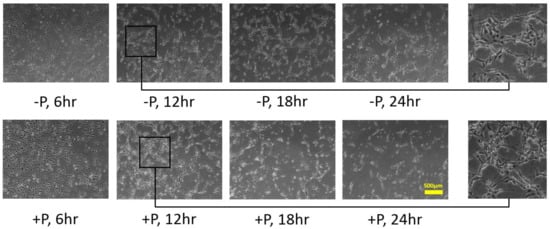
2.1. HUVECs Cultivation on Type I Collagen and Fibronectin
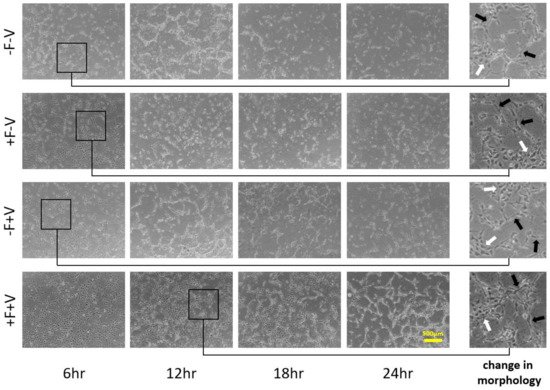
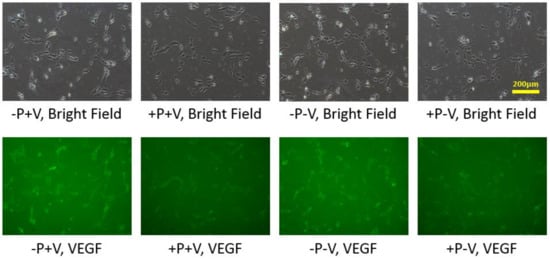
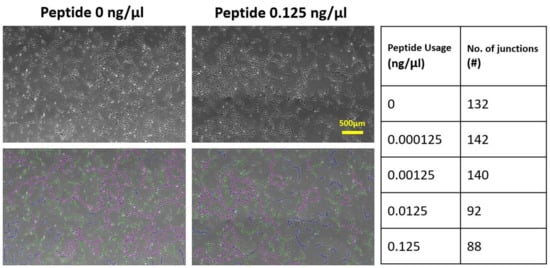
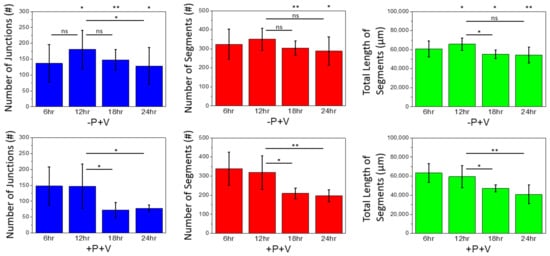
2.2. Effects of Soluble and Bound VEGF
3. Current Insights
This entry is adapted from the peer-reviewed paper 10.3390/app11209501
References
- Prakash Vempati; Aleksander Popel; Feilim Mac Gabhann; Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine & Growth Factor Reviews 2013, 25, 1-19, 10.1016/j.cytogfr.2013.11.002.
- J E Park; G A Keller; N Ferrara; The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF.. Molecular Biology of the Cell 1993, 4, 1317-1326, 10.1091/mbc.4.12.1317.
- Jeremy Grunstein; Joseph J. Masbad; Reed Hickey; Frank Giordano; Randall S. Johnson; Isoforms of Vascular Endothelial Growth Factor Act in a Coordinate Fashion To Recruit and Expand Tumor Vasculature. Molecular and Cellular Biology 2000, 20, 7282-7291, 10.1128/mcb.20.19.7282-7291.2000.
- Tom T. Chen; Alfonso Luque; Sunyoung Lee; Sean M. Anderson; Tatiana Segura; M. Luisa Iruela-Arispe; Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. Journal of Cell Biology 2010, 188, 595-609, 10.1083/jcb.200906044.
- Christiana Ruhrberg; Holger Gerhardt; Matthew Golding; Rose Watson; Sofia Ioannidou; Hajime Fujisawa; Christer Betsholtz; David T. Shima; Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes & Development 2002, 16, 2684-2698, 10.1101/gad.242002.
- Errol S. Wijelath; Salman Rahman; Mayumi Namekata; Jacqueline Murray; Tomoaki Nishimura; Zohreh Mostafavi-Pour; Yatin Patel; Yasuo Suda; Martin Humphries; Michael Sobel; et al. Heparin-II Domain of Fibronectin Is a Vascular Endothelial Growth Factor-Binding Domain. Circulation Research 2006, 99, 853-860, 10.1161/01.res.0000246849.17887.66.
- Mikaël M. Martino; Federico Tortelli; Mayumi Mochizuki; Stephanie Traub; Dror Ben-David; Gisela A. Kuhn; Ralph Müller; Erella Livne; Sabine A. Eming; Jeffrey A. Hubbell; et al. Engineering the Growth Factor Microenvironment with Fibronectin Domains to Promote Wound and Bone Tissue Healing. Science Translational Medicine 2011, 3, 100ra89-100ra89, 10.1126/scitranslmed.3002614.
- Valerie L. Cross; Ying Zheng; Nakwon Choi; Scott S. Verbridge; Bryan A. Sutermaster; Lawrence J. Bonassar; Claudia Fischbach; Abraham D. Stroock; Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596-8607, 10.1016/j.biomaterials.2010.07.072.
- Katerina Stamati; John V. Priestley; Vivek Mudera; Umber Cheema; Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake. Experimental Cell Research 2014, 327, 68-77, 10.1016/j.yexcr.2014.05.012.
- Alvaro Köhn-Luque; Walter De Back; Jörn Starruß; Andrea Mattiotti; Andreas Deutsch; José María Pérez-Pomares; Miguel A. Herrero; Early Embryonic Vascular Patterning by Matrix-Mediated Paracrine Signalling: A Mathematical Model Study. PLoS ONE 2011, 6, e24175, 10.1371/journal.pone.0024175.
- A Köhn-Luque; Walter De Back; Y Yamaguchi; K Yoshimura; M A Herrero; T Miura; Dynamics of VEGF matrix-retention in vascular network patterning. Physical Biology 2013, 10, 066007, 10.1088/1478-3975/10/6/066007.
- Roeland M. H. Merks; Erica D. Perryn; Abbas Shirinifard; James A. Glazier; Contact-Inhibited Chemotaxis in De Novo and Sprouting Blood-Vessel Growth. PLOS Computational Biology 2008, 4, e1000163, 10.1371/journal.pcbi.1000163.
- Patrick Namy; Jacques Ohayon; Philippe Tracqui; Critical conditions for pattern formation and in vitro tubulogenesis driven by cellular traction fields. Journal of Theoretical Biology 2004, 227, 103-120, 10.1016/j.jtbi.2003.10.015.
- Patterson, J.; Martino, M.M.; Hubbell, J.A.; Biomimetic materials in tissue engineering. Materials Today 2010, 13, 14-22, .
- Amanda K. A. Silva; Cyrille Richard; Michel Bessodes; Daniel Scherman; Otto-Wilhelm Merten; Growth Factor Delivery Approaches in Hydrogels. Biomacromolecules 2008, 10, 9-18, 10.1021/bm801103c.
- Karl H. Plate; Georg Breier; Herbert A. Weich; Werner Risau; Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359, 845-848, 10.1038/359845a0.
- Vladimira Moulisova; Cristina Gonzalez-García; Marco Cantini; Aleixandre Rodrigo-Navarro; Jessica Weaver; Mercedes Costell; Roser Sabater i Serra; Matthew Dalby; Andrés J. García; Manuel Salmerón-Sánchez; et al. Engineered microenvironments for synergistic VEGF – Integrin signalling during vascularization. Biomaterials 2017, 126, 61-74, 10.1016/j.biomaterials.2017.02.024.
- Jessica D. Weaver; Devon M. Headen; Jahizreal Aquart; Christopher T. Johnson; Lonnie D. Shea; Haval Shirwan; Andrés J. García; Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Science Advances 2017, 3, e1700184-1700184, 10.1126/sciadv.1700184.
- J. F. Dye; L. Lawrence; C. Linge; L. Leach; J. A. Firth; P. Clark; Distinct Patterns of Microvascular Endothelial Cell Morphology Are Determined by Extracellular Matrix Composition. Endothelium 2004, 11, 151-167, 10.1080/10623320490512093.
- Tania R. Chan; Patrick J. Stahl; Yang Li; S. Michael Yu; Collagen–gelatin mixtures as wound model, and substrates for VEGF-mimetic peptide binding and endothelial cell activation. Acta Biomaterialia 2015, 15, 164-172, 10.1016/j.actbio.2015.01.005.
- Ke Xu; Ondine Cleaver; Tubulogenesis during blood vessel formation. Seminars in Cell & Developmental Biology 2011, 22, 993-1004, 10.1016/j.semcdb.2011.05.001.
