4. Discussion
The presence of fungi in an indoor environment is influenced by a wide range of variables, such as human occupancy and their activities, humidity levels, ventilation, environmental characteristics, water infiltrations, building and decoration materials and outdoor air [
25,
26]. Furthermore, there is strong scientific evidence corroborating the relationship between the building dampness, visible mould, and moisture damage with adverse respiratory health effects [
4,
27,
28,
29,
30,
31,
32]. Moisture, nutrients and temperature are proved to be the most important variables that influence the growth and dissemination of fungi on building materials [
33]. Thus, the results regarding
Aspergillus sp. and
Fumigati section contamination in the assessed FFHs were the expected considering the observed FFH conditions (in 8 from the 11 FFHs): leakages, visible mould growth and cracks on the walls/floor.
The identification of the
Aspergillus section
Fumigati through passive and active sampling has already been reported [
4,
34,
35,
36,
37,
38]. However, other studies developed in Portuguese occupational environments presented a lower prevalence of
Aspergillus sp. and, more specifically, section
Fumigati [
9,
10,
12,
13,
35]. Indeed, in primary health care centres the
Fumigati section presence was 33.3% on surface swabs and 1.3% in EDCs [
9,
10]. In one central hospital,
Aspergillus sp. presented an overall prevalence of 17.25%, with the
Fumigati section only being observed in the vacuum bag [
12]; in Portuguese bakeries, the
Fumigati section was found on air samples (3.2% on DG18) and in EDCs (8.3% on MEA and 50% on DG18) [
13]; in three fitness centres, only four isolates of this section were found in one air sample [
35].
In this study, a multiple approach protocol was performed comprising the two sampling methods for a better characterization of contamination in FFHs.
Aspergillus counts were revealed to be higher in settled dust filters and cleaning cloths. Previous studies also identified passive sampling as suitable to determine
Aspergillus section
Fumigati by culture-based methods through sampling of filtering respiratory protective devices and other environmental matrices in settings such as waste-sorting plants, veterinary clinics, dairies, ambulances, and many other indoor and occupational environments [
4,
10,
16,
18,
32]. In fact, passive sampling is able to characterize contamination levels over a wider period of time, compared to air sampling [
21,
36]. Thus, higher fungal counts and greater fungal diversity are expected by passive sampling.
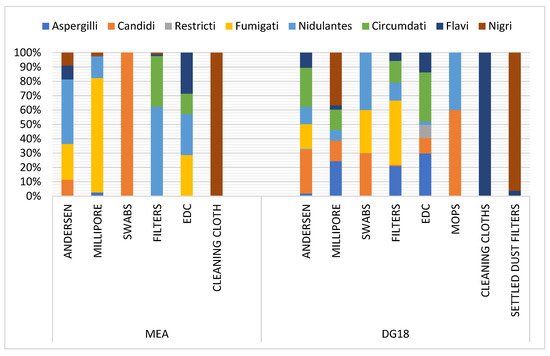
The
Aspergillus section
Fumigati was predominant in swabs and settled dust filters in DG18, and in EDCs in MEA, suggesting that reliable matrices for
Aspergillus section
Fumigati exposure assessment were chosen [
4]. The significant differences in fungal counts between passive and active sampling highlight the advantages associated with a multi-approach protocol that comprises active and passive sampling simultaneously, overcoming the limitations associated with each sampling method [
4,
7,
9,
10,
11,
12,
13,
14,
15,
16,
39].
Regardless of the lower prevalence of
Aspergillus sp. in air samples performed by Millipore and six-stage Andersen, the
Fumigati section was predominant in Millipore air samples in MEA. Furthermore, the underestimation of microbial contamination collected by impaction devices (Andersen six-stage and Millipore sampling devices), due to cell damage during sampling process, has already been stated [
18,
40,
41]. However, the six-stage Andersen sampler allows the hazardous range where the
Fumigati section has lung penetrability to be identified [
17,
18,
41]. Indeed, in four of the assessed FFHs, section
Fumigati at stage 6 (0.65 µm) of reaching alveoli was observed. This has the potential to cause respiratory diseases (inflammation activation) by activating macrophages, B cells and T cells [
42]. The same concern was raised in a study held in Portuguese ambulances used in emergency clinical services [
18].
Aspergillus section
Fumigati was more frequent in DG18 compared to MEA counts. Despite the recommendation of using MEA for aerobiological studies in a Portuguese regulatory framework dedicated to the assessment of indoor air quality (IAQ) (25APA 2010), DG18 is an efficient option due to its restrictive character, inhibiting the development of fastidious fungal species [
21]. The results of some fungal overgrowth on the MEA plates may have influenced the development of
Aspergillus sp. and, more specifically, of the
Fumigati section, due to chemical competition [
43], highlighting the use of both culture media for a wider fungal characterization [
4,
7,
9,
10,
11,
12,
13,
14,
15,
16,
39]. Sample dilution prior to inoculation (to avoid the excessive development of fast-growing fungi on media plates) was not performed due to the limitations of this option. Indeed, the removal of rare types of organisms leads to differences in species richness and diversity, decreasing competition among microorganisms, causing a probable overgrowth in some species that were not as prevalent in the original community [
44].
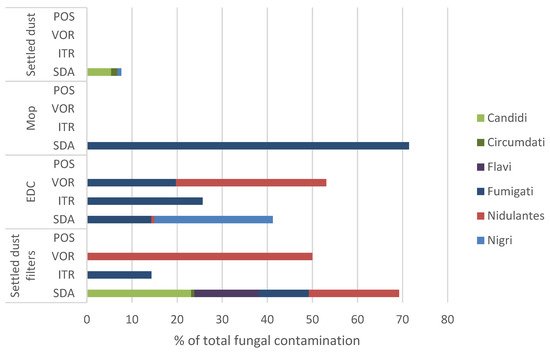
The higher mean rank values obtained for total fungal contamination,
Aspergillus sp. counts, and
Aspergillus section
Fumigati with SDA and ITR+VOR media, compared to MEA or DG18, suggest Sabouraud is a more suitable media for the recovery of fungi and
Aspergillus sp. The use of Sabouraud as a standard medium to assess outdoor airborne fungi by air sampling was generally supported in a recent study on media comparison [
45], and has also been supported in clinical applications [
46]. However, Saboraud enhanced
Chrysonilia sitophila in other performed assessments [
9,
18].
The ability to use protection against respiratory devices or filters as a sampling approach depends on the features of the assessed setting, activities developed and duration of use, e.g., mop sampling depends on cleaning procedures. The EDC device, on the other hand, allows for the recovery of fungal contamination in a consistent and standardized manner (regarding retention material and collection period), and it is a low-cost, low-maintenance sampling strategy that has been increasingly used in the assessment of occupational exposure to fungal burden [
19] and in indoor air quality studies [
21,
32].
The fact that, among
Aspergillus sections, only the
Fumigati section was found in azole-supplemented media, confirms the presence of fungi potentially resistant to azoles in FFHs. If this azole-resistance phenotype is further confirmed by molecular analysis or antifungal susceptibility testing, it might represent a health risk for workers in this setting, especially in the FFHs where contamination by
Aspergillus section
Fumigati was higher. This health risk arises from the fact that azole-resistant fungi might cause invasive infections, especially in immunocompromised individuals, which are of difficult control due to the limited treatment options [
2,
4,
8,
19]. In addition to being the etiological agent of invasive aspergillosis, the
Fumigati section is also responsible for more common respiratory symptoms such as asthma, allergic sinusitis, cough and bronchial hyperresponsiveness [
47].
Culture-based methods allowed the
Aspergillus section
Fumigati to be identified in various matrices (settled dust filters, swabs, EDCs and air samples from Millipore and Andersen), confirming the results of molecular detection in EDCs and filters. The use of qPCR further enabled the
Fumigati section to be detected in additional matrices (identification badges, mops and cleaning cloths) where it was undetectable by culture. This may be associated with the absence of fungal viability due to an impediment to grow in culture (e.g., due to competition for nutrients), while the molecular tools enable even non-viable microorganisms to be identified [
48]. Failure to detect the
Aspergillus section
Fumigati by qPCR in swabs and air samples (in contrast to the results obtained by culture) may be associated with ineffective DNA extraction in sample processing, or the presence of inhibitors (such as particles from air samples), misleading the results [
4,
49,
50]. Without diminishing the advantages of molecular analysis, classical culture-based methods are still necessary to assess the viability of pathogenic microorganisms related to their infectivity potential. Indeed, a microorganism’s viability is associated to the potential of inflammatory and cytotoxic responses and, consequently, the infection potential. Therefore, molecular tools must be used in parallel with classic methods [
4,
51].
Correlation was found in this study between total fungal counts,
Aspergillus sp. counts and
Fumigati section counts not following the trend previously found in health care environments [
9]. This means that the measures used to avoid fungal contamination in this setting are also effective concerning
Aspergillus contamination. Nevertheless,
Aspergillus genera assessment should always be performed, as specific
Aspergillus sections (
Flavi, Fumigati, Circumdati and
Nidulantes) are indicators of harmful fungal contamination when found on air samples and require intervention, as referred to by the American Industrial Hygiene Association and Portuguese regulatory framework concerning IAQ [
25,
52].
Thus, considering the lack of scientific information in this specific environment, further studies are needed to characterize the overall exposure to fungal contamination and other microbiological agents, as well as regarding the most suitable corrective and preventive measures used to avoid exposure. Additionally, further research on azole-resistance profile must be conducted to better estimate the risk of exposure to resistant Aspergillus section Fumigati in this setting, namely, screening azole resistance at selective conditions for Aspergillus section Fumigati, molecular analysis of resistance mutations, and antifungal susceptibility testing.