Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
The gravity environment in space is termed “microgravity” (μG) and is defined as approximately 10−6 of Earth’s surface gravity (G), as there is never truly a complete absence of gravity. The effects of μG on various cell types have been documented in bone, cartilage, and endothelial cells, to name a few, and immune cells are no exception. Among the various microgravity-induced side effects, a compromised or altered immune response can have serious consequences and jeopardize the survival of humans in space.
- microgravity
- T cells
- altered gravity
- cell signaling
- immunity
- space
- immune response
- weightlessness
- apoptosis
- activation
1. The Importance of Human Health and Immunity for Space Exploration
As we enter the new decade, humans are as focused as ever on the exploration of space. The rationale of such a profound goal lies in the potential for long-term colonization on planets besides Earth. The National Aeronautics and Space Administration’s (NASA) “Artemis” program aims to reach the Moon again by 2024, which is the first step to landing a human on Mars [1]. This would require human astronauts to potentially live in space for years, something that has not been attempted to this day. Such an endeavor comes with perplexing problems including a lack of sufficient knowledge on how the human body adapts and reacts to the microgravity environment. Thus, preparing for possible biological and psychological complications caused by deep-space exploration is a priority for space research programs.
From the days of the first Apollo missions to the moon, it has been observed that astronauts suffer from physiological changes due to the challenging environment in space, with which they are unacquainted [2,3,4]. Such changes include, but are not limited to, bone density loss [5,6,7], muscle atrophy [8,9,10], impaired eyesight [11], cardiac dysfunction [12], loss of proprioception [13], immune dysregulation [14,15], and changes in the expression of many genes [16,17]. These changes can be attributed to numerous factors in space such as radiation, disrupted circadian rhythm, and most of all the lack of Earth’s gravity, which has played a pivotal role in determining baseline development and homeostasis since the beginning of human evolution.
The gravity environment in space is termed “microgravity” (μG) and is defined as approximately 10−6 of Earth’s surface gravity (G), as there is never truly a complete absence of gravity [18]. The effects of μG on various cell types have been documented in bone, cartilage, and endothelial cells, to name a few, and immune cells are no exception [5,6,19,20]. Among the various microgravity-induced side effects, a compromised or altered immune response can have serious consequences and jeopardize the survival of humans in space. Therefore, understanding how μG affects the functions and components of the immune system over short or long periods is very crucial.
The human immune system is a complex interdisciplinary network that incorporates a wide host of cells and molecules which maintain the organism’s health. It serves to protect the human body against malignant tissues and exogenous factors such as pathogens. It is broken into two branches: innate and adaptive immunity. Innate immunity does not require prior exposure to a pathogen to activate a swift and nonspecific response, whereas adaptive immunity requires previous exposure to a pathogen in order to initiate a specific response [21]. Innate immunity includes the physical barriers of the skin and mucous membranes that prevent the entry of pathogens into the body. The innate immune system also includes defensive cells that protect the body against pathogens that pass the barriers. These cells include natural killer cells, dendritic cells, neutrophils, eosinophils, basophils, mast cells, and the monocyte/macrophage system. Adaptive immune response occurs in conjunction with the innate immune system in order to eliminate pathogens. It is made up of T lymphocytes, B lymphocytes, antibodies in the blood, and messenger cytokines in the blood and tissue [22].
2. Mechanisms Involving Microgravity-Induced Changes in T Cell Signaling Pathways
Cell signaling is perhaps the most important method of communication in the body [106,107]. Signal transduction is a part of nearly every cell and mediates everything from cellular production to life cycle [26]. Through a series of molecules and receptors, signals can directly alter genetic expression and therefore cell function. Cells take cues from their neighboring cells and the environment to determine what they do and how they do it. A system so perceptive is also just as sensitive; thus, changes of the upstream signal may lead to large chain reaction responses [108]. For this reason, signal transduction is an intriguing subject of study with regard to μG. An extreme and unpredictable change such as the removal of gravity-induced loading may exert its overall effects through cell signaling pathways. T cells were chosen as the topic of investigation because of their large role in adaptive immunity. These lymphocytes are directly responsible for the adaptive immune response, an important process in keeping humans protected from repeat exposure. A review of current literature revealed that innate immune signaling, modeled by macrophages, has recently been investigated in microgravity [109]. Additionally, there is little experimental evidence about the effect of μG on other immune cell types, such as Natural Killer Cells. Currently, there has not been an extensive review of specific mechanistic changes in adaptive T cell signaling in μG such as these.
T cells recognize and destroy infected cells by using special surface structures that can bind specific pathogens. T cells also have the ability to remember previous antigens; this allows for an efficient response to known antigens. In the body, T cells have a number of functions, from killing infected cells to acting as messengers when recruiting more immune cells to respond to a stimulus. During a response, T cells can develop into specialized cells such as helper T cells, cytotoxic T cells, memory T cells, and regulatory T cells [22]. It is important to note that some studies show variable results in experiments performed under μG conditions on T cell precursors [110,111], on populations of these specialized T cells [44,49,105,112,113], and on the distribution of T cells in the body [103,114]. However, most of the studies that we investigated have broadly used T lymphocytes as a model for adaptive immunity, obtained from a variety of sources. To understand adaptive immunity in μG, the signaling pathways regarding T cells should be considered. Specifically, activation and apoptotic pathways of T cells are especially important in understanding general adaptive immune functions.
The T cell activation pathway can begin by two general means in vivo: direct interaction with an Antigen-Presenting Cell (APC), and activation via Interleukin-2 (IL-2). Although most μG studies use antigens such as phorbol myristate acetate (PMA), phytohaemagglutinin (PHA), or Concanavalin A (ConA) as stimulation methods, there are a few that review APC interactions via dendritic cells or via the effects of the monocyte/macrophage system on T cells by looking at levels of tumor necrosis factor alpha (TNF-a) [62,71,76,86]. Direct activation requires the presented antigen to form a complex with the naive T cell Receptor (TCR), along with CD28 co-stimulation and cytokine signaling. Alternatively, IL-2 secretion from the initial cell may begin the cascade of auto-proliferation and proliferative signaling of other T cells [115]. This delicate activation pathway may very well be changed by μG and significantly affect immune regulation. Figure 1 displays the T cell “flowchart”, an overall scheme to compare inputs and outputs of T cell activation. Different stimulation methods (inputs) and their targets can be seen on the left side of the image, while activation responses (outputs) are depicted on the right side. The initial T cell is directly activated and begins an amplificatory, proliferative cascade to other T cells through IL-2, among other pro-inflammatory cytokines. This amplification reaction also includes macrophages and other immune cells to complete the immune response.
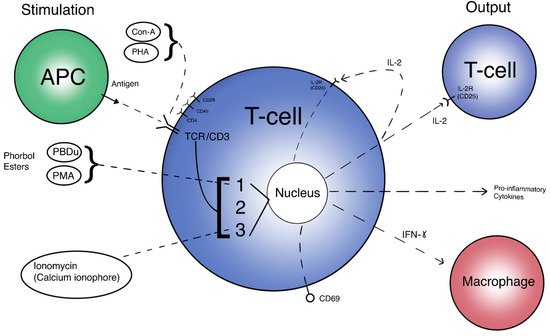
Figure 1. A simplified view of T cell activation. Inputs are different methods of stimulation and their respective targets. Outputs are expressed proteins and molecules targeting other immune cells. 1 = PKC/NF-kB pathway. 2 = Ras/AP-1 pathway. 3 = Calcineurin/NFAT pathway.
Normal activation is needed for proper adaptive immune defense; lack of T cell activation leads to impaired immune response. The consequences of impaired immunity include infection by pathogens and possibly unchecked neoplastic growth, which may lead to illness and potentially death. Apoptosis is also an important factor in T cell immunity, as pre-planned cell death lends itself to immune system efficiency. Specific cell death pathway alterations due to μG may affect the overall normal function of T cells.
2.1. Signaling Pathways Responsible for T Cell Activation under Microgravity
It has been well documented that T cell activation and its proliferation are downregulated in μG [52,64,66,88,90,100]. As such, investigations primarily aim to uncover the interruption in cell signaling that leads to the limited activation levels in T cells. One of the most identifiable intercellular signaling molecules is IL-2, a cytokine essential for early T cell activation. IL-2 is the most widely accepted and commonly used method to test T cells for activation response, dating back to early studies. It acts as a direct measure of T cell activation, production, and signal amplification. While other pro-inflammatory cytokines may also be tested, none are as directly tied to the T cell activation cascade as IL-2, and therefore not as used in the literature of interest. Many studies have demonstrated that IL-2 production and the IL-2 receptor (IL-2R/CD25) are decreased in real and simulated μG conditions [46,51,52,64,67,68,69,88,90,97,101,116]. For instance, Chang et al. conducted experiments on the space shuttle Discovery and showed that IL-2 secretion in mice was decreased after a 15-day long μG exposure as compared to counterparts on Earth [101]. The decrease in IL-2 suggests that the in-flight mice have depressed T cell activation response in the absence of gravity. Besides in-flight studies, the studies conducted under a simulated gravity environment demonstrated decreased IL-2 expression. Boonyaratanakornkit et al. studied T cells isolated from peripheral blood leukocytes using 3D clinostat. They demonstrated that 4 h of μG exposure decreased IL-2 expression [67]. Another study conducted with RWV for 24 h proved that μG decreased expression of IL-2 in both CD4+ and CD8+ T cells [64]. Interestingly, CD4+ T cells were more sensitive to μG changes than CD8+ T cells, as evidenced by IL-2 levels. Taken together, it is reasonable to assume μG has a suppressive effect on IL-2 in the T cell activation pathway.
IL-2R/CD25 is just as important as IL-2 in determining the efficacy of T cell activation. The expression of cell surface receptors directly influences downstream pathways. IL-2R is shown to be downregulated in both real and simulated μG via parabolic flight and 2D clinostat, respectively [51]. In both cases, T cells activated with Con-A and anti-CD28 showed rapid suppression of IL-2 in μG. In addition, testing of CD3 (a very early-pathway cell surface receptor) expression also showed evidence of downregulation in μG. Decreased expression of TCR/CD3, or any of its subunits, on the cell surface would lead to significant impairment of the activation signaling cascade in T cells, as it is one of the first steps that initiates activation. This suggests that T cell activation receptors are inhibited throughout the pathway. Similarly, in an aforementioned study, IL-2R expression was confirmed to be downregulated in μG after Con-A anti-CD28 activation [67]. IL-2R was again shown to be downregulated in μG via 2D clinostat and shuttle spaceflight after activation by combinations of PMA, Leu4, PHA, and ionomycin [52]. Both modeled μG and real μG under the several listed stimulation methods proved the wide range and effect of μG on T cell activation, as shown by depressed IL-2R and CD69 levels. In a study by Martinez et al., both lL-2 and IL-2R were confirmed to be decreased in activated T cells in μG as well [69]. The downregulation of IL-2 paired with decreased IL-2R/CD25 receptor expression contributes to the depressed activation and proliferation of T- lymphocytes.
The expression of IL-2 and its receptor are indicative of activated T cells later in the activation pathway. However, the regulation of IL-2 and other cytokine signals are controlled at some upstream signaling pathways [26]. Thus, in order to understand how IL-2 secretion is affected and this subsequently affects T cell activation, we need to focus on the three important signaling pathways: PKC/NF-kB, Ras/AP-1, and Calcineurin/NFAT. Figure 1 depicts a broad, extracellular look at these pathways and how they are activated in the overall function of the activated T cell. Figure 2 provides a more detailed look at the relationship between these pathways intracellularly. Each activation pathway works in conjunction with the others and ends in a transcription factor that helps to express relevant proinflammatory genes (such as IL-2 and other cytokines, seen in Figure 1 outputs). Inhibition or dysregulation at any point in these pathways, or upstream from them will be responsible for IL-2 reduction and the subsequent suppression of T cell activation in μG. We will investigate which steps in these pathways may be impacted by microgravity.
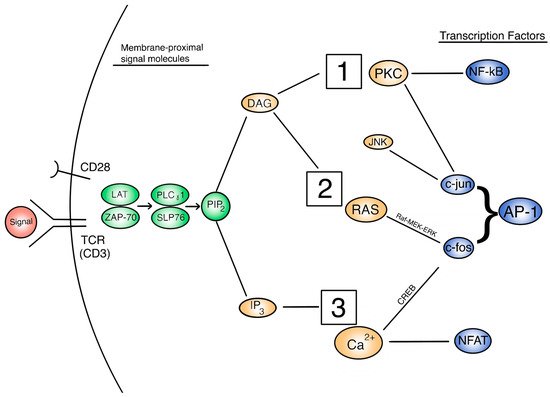
Figure 2. A view of the intracellular T cell activation signal cascade, with signal molecules of interest. 1 = PKC/NF-kB pathway. 2 = Ras/AP-1 pathway. 3 = Calcineurin/NFAT pathway.
2.2. Signaling Pathways Responsible for T Cell Apoptosis under Microgravity
Apoptosis has many homeostatic purposes in cells of the human body [26,127]. In the immune system, programmed cell death is important for immune response, tumor suppression, and cytotoxic killing [128]. It has been observed that apoptosis is significantly increased in T cells subjected to μG [78,85,89,92,101,129,130,131,132,133]. Fas/APO-1 (also known as FasR or CD95) is a cell surface receptor and is an important regulator of apoptosis in T cells, and is studied because it is the best-defined factor of apoptosis signaling [129]. The interaction of Fas and Fas ligand (Fas-L) constitutes the beginning of the apoptotic pathway. In a study of OVA-stimulated mouse splenocyte T cells subjected to real μG during spaceflight, there was a larger number of cells in the ground control versus spaceflight [101]. To investigate the presence of apoptotic upregulation, Fas-L relative expression was measured and observed to be increased in the μG treated group. This suggests that an increase of Fas-L correlates to more apoptosis in-flight versus 1G ground when unstimulated (though stimulated cells did not have a significant enough change).
In a similar experiment that utilized a space shuttle to provide real μG to Jurkat T cells, it was observed that apoptosis was markedly increased compared to 1G controls [129]. By utilizing DNA condensation staining, it was found that there was 28% apoptosis in μG and only 12% apoptosis in ground control. This suggests that apoptosis is significantly upregulated (over two times as much) because of μG. To verify the involvement of Fas/APO-1, an ELISA kit was used to measure expression levels at different time increments. It was observed that Fas expression was increased by 15× between 4 and 24 h, and 65× at 48 h. This proves that Fas is significantly increased in a time-dependent manner in μG. Putting the results of this study together, we can conclude that the increase in Fas likely correlates with the upregulated apoptosis when subjected to μG.
Increased levels of Fas/CD95 were further exhibited as a result of modeled μG in CD8+ T cells, stimulated by Con-A in a RWV experiment [64]. Also, late apoptotic CD4+ and CD8+ T cells were observed to increase in μG compared to controls. Although the significance of CD4+ T cells was not established for the Fas expression assay, the overall data corroborates the proposition that the Fas signaling step of apoptosis is directly affected by μG. In another study, T cells subjected to μG via spaceflight and activated by increasing FBS concentration and temperature showed an increase in Fas levels and subsequent apoptosis [130]. Specifically, the number of Fas positive cells was increased in μG at 48 h compared to ground samples, tested by immunofluorescence microscopy. Also, Fas levels were expectedly time-dependent and increasing in μG. The percent of apoptotic cells also increased significantly in μG as compared with ground controls, correlating with Fas expression levels. Interestingly, this study also showed that cell density was not a factor in determining cell viability/apoptosis in space-flown cell cultures, strengthening the argument that μG impairs cell viability by inducing apoptosis. Based on these studies, it can be reasonably said that Fas/APO-1 receptor (CD95) is upregulated in T cells under real and simulated μG and leads to an increase in apoptosis [129,130,131]. We should thus shift the investigation to what causes the increased levels of these (and other) apoptotic molecules.
Apoptotic signaling is broken into a few notable pathways, as shown in Figure 3. Firstly, the Fas-L mediated pathway (known as the extrinsic pathway) is immediately responsible for inducing apoptosis by signaling other cells and self-signaling, as noted earlier [134]. However, the signaling cascade that leads to the expression of Fas in the first place should be our primary interest. Fas-L is thought to be produced as a result of over-activation as a means of shutting down hyperactive cells, though Fas/APO-1 is constitutively expressed in activated T cells [129]. Alternatively, the apoptotic cascade can be initiated by the intracellular (intrinsic) pathway. This pathway is sparked by the cell’s perception of stress, be it by physical damage, radiation, heat, or other processes. This pathway is normally inhibited by Bcl-2, and suppression of this protein can lead to uncontrolled or increased apoptosis [135].
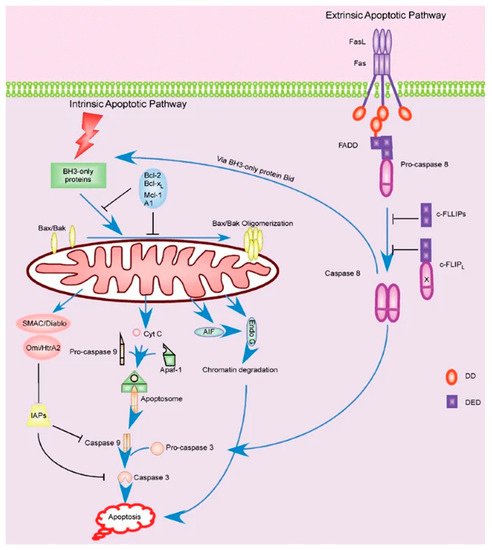
Figure 3. Extrinsic and intrinsic apoptotic pathways within the T cell [134].
Bcl-2 is one of the most important modulators of the apoptotic pathway. It is normally present in high levels in actively working cells. Apoptosis can be induced if Bcl-2 is suppressed in some manner. Sokolovskaya et al. investigated the behavior of Bcl-2 in T cells under μG via RPM. They observed that Bcl-2 remained unchanged in the μG group of non-activated Jurkat T cells compared to the 1G controls [131]. In a similar study, anti-CD3 activated T cells in a RWV also showed that Bcl-2 was unchanged in μG compared to 1G [136]. The study also measured the levels of BAX, which along with BAK, is an important pro-apoptotic molecule along the intrinsic pathway. It was observed that these levels did not change in the μG condition. It is important to note that Fas and Fas-L were both also unchanged in this same study, which contradicts many of the previous studies and may invalidate part of the results. In another RWV study, it was shown that both Bcl-2 and BAX were downregulated in T cells under μG conditions [137]. Taken as a whole, it may be possible that the intrinsic pathway is not significantly affected in any meaningful way by μG.
Apoptosis can be an outcome of cell stress if the perceived damage is too great, but cells have mechanisms in place to survive through some stressors. One of the most important of these mechanisms is Heat Shock Protein (HSP). HSPs are a family of proteins that can activate signaling cascades and cell functions when they sense the cell is under stress, including helping in protein folding, chaperoning, and cell proliferation [138]. HSPs also inhibit bid and cytochrome c, which are both pro-apoptotic factors [26]. In a study by Novoselova et al., it was observed that HSP-72 and HSP-90 were both increased in non-activated mouse T cells after 12 h and 7 days in spaceflight, respectively, compared to ground controls [85]. However, it should be noted that only postflight samples were taken, which does not control for take-off and landing stress. In another HSP study, it was found that HSP-70 was significantly increased, but HSP-90 was significantly decreased [27]. It is expected that all HSPs should increase due to stress; however, the details of microgravity-induced cellular stress are relatively unknown. The study suggests that μG may have a very specific effect on HSPs individually. Additionally, it was observed that HSP-70 remained unchanged in the previously discussed study by Sokolovskaya et al. [131]. Taken together, there is conflicting evidence about the effect of μG and how it influences the levels of HSPs. More research should be done to further elucidate the behavior of HSP regulation in μG.
Although apoptosis is an important method of immune system (specifically T cell) dysfunction in μG, it has not been as well studied as its importance may suggest. There has yet to be conclusive evidence exposing the mechanisms behind increased apoptosis, other than the increase in Fas/APO-1 expression. However, the observation that apoptosis in T cells is increased in μG is well established in itself. We posit that due to this increase in cell death and subsequent decrease in viable cells in μG conditions, testing for activation of T cells may be easily skewed. Many activation-related studies do not control for an increase in apoptosis, thereby potentially decreasing the number of activation products and implying that the activation suppression is worse than if it were just due to cell signaling disruption. To this end, it is crucial to control for both apoptosis and activation in experiments in order to allow for a greater understanding of overall T cell behavior in μG.
This entry is adapted from the peer-reviewed paper 10.3390/life11101043
This entry is offline, you can click here to edit this entry!
