Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
Polymers as smart temperature sensors
- thermochromism
- polymers
- intelligent packaging
- thermal sensor
1. Prospective Polymer Candidates
1.1. Polydiacetylene (PDA)
Conjugated polymers are materials which have the potential to be used in diverse applications in interdisciplinary areas due to their structural, spectral, and optical characteristics. A good example of this is polydiacetylene (PDA) The feature of interest in this study is their unique colorimetric and fluorescent transitions they undergo in response to an external stimuli (temperature) [14]. Unlike most of their other counterparts, polydiacetylene can undergo polymerization without the help of chemical initiators, catalysts and high temperatures. This is important as polymerization allows for simple processing of diacetylene [14]. However, this is not the most important aspect of PDAs. The absorption peak of approximately 640nm, due to electron delocalization, appears optically as a blue color. This blue shift is caused by the conformational change of the polymer backbone from planar to nonplanar [14]. The first known PDA based sensor was created in 1993 by Charych et al. [15]. They created a selective sensor for the influenza virus. However, PDAs are being used in a vast number of applications such as cell imaging, tumor targeting and now, in this study, as a thermal sensing system.
During a change in temperature, PDAs undergo a colorimetric transition from blue to red. Nevertheless, the most important aspect of PDAs is their reversibility within a certain temperature range. This property is usually achieved by strengthening their intermolecular interactions.
This study focuses on the use of thermochromic polymeric sensors in food packaging applications. An ideal candidate is a polymer which provides a clear visual color change without the need for wires or other complicated appendages that are usually attached to thermocouples for temperature monitoring. Consequently, PDA was one of the candidates that was considered in the temperature sensing system. This is because of the previous work done by Huo et al. which shows that PDAs can be modified to present a color change over a wide range of temperatures up to 250 °C while maintaining thermal stability. Huo et al. studied different compositions of PDAs. These PDAs (labelled 2a,2b,2c) were obtained via the Glaser reaction using a diacetylene moiety as a polymerizable core, amino acid moiety as a linking agent and two pyrene units as bilateral head group [16]. Figure 1 depicts the different PDAs (labelled 2a,2b,2c) that were made.
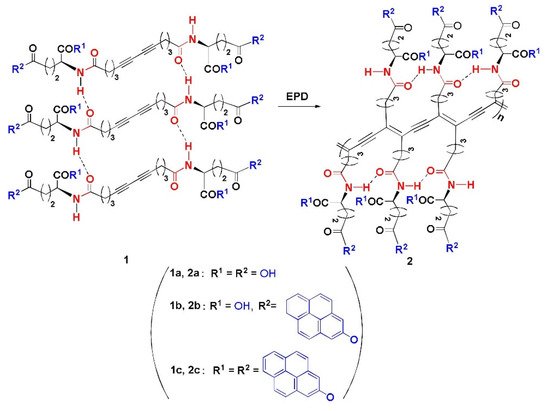
Figure 1. The EPD (electrophoteric deposition) process of polymeric products 2a–2c. Reproduced with the permission of Reference [16]. Copyright 2017, Elsevier.
Thermogravimetric analysis was performed on the PDAs between 25 and 800 °C and they were observed to maintain their stability up to temperatures as high as 300 °C. The results can be seen in Figure 2.
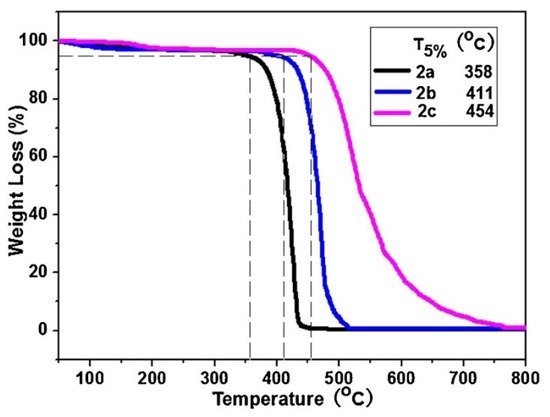
Figure 2. Thermogravimetric analysis of polymers, 2a–2c, collected under a N2 atmosphere. Reproduced with the permission of Reference [16]. Copyright 2017 Elsevier.
Thermochromic Transitions in Polydiacetylene.
Thermochromism in PDAs was investigated by heating blue polymer films at 5 °C min−1 under vacuum and the visible color change observed is presented in Figure 3. Real time monitoring was performed using a fiber optic absorption spectrometer. It was observed (Figure 3) that the color change was reversible. For example, in PDA 2a, a shift in the original blue color at 25 °C to a red at 250 °C and a change back to blue when it is cooled to its original temperature is observed. The PDA 2b shows a similar change but the most distinct color change comes from the PDA 2c at 300 °C.
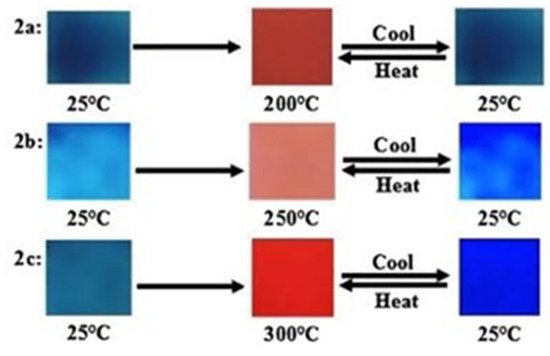
Figure 3. Photographs of the PDAs 2a–2c recorded upon increasing temperature from 25 °C to 300 °C and then cooling to room temperature. Reproduced with the permission of Reference [16] Copyright 2017 Elsevier.
Yoon et al. also explored PDA. They developed diacetylene (DA) supramolecules that are inkjet printable which makes it highly advantageous in terms of ease of printability on large surfaces in a high throughput manner as well as its low production cost along with low waste materials produced [17]. Paper was used as the substrate which makes it suitable for not only scientific analysis but also in daily life applications [17]. A single component ink system that was compatible with the ink cartridge nozzle was created. It was composed of DA, bisurea and oligoethylene oxide moieties [17]. The DA monomers generated PDA when it was irradiated with ultraviolet rays. The other components enhanced supramolecular assembly of the monomers and increased water compatibility respectively [17].
DA 1-DA 3 were prepared by an increase in the number of ethylene oxide units. It increased from 4 (DA 1) to 7 (DA 2) and to ca. 10 (DA 3). Consequently, this causes a corresponding enhancement in the hydrophilic property of the monomer. However, in the case of DA 3, the exact number of the ethylene oxide group is unknown because it is prepared from commercially available poly (ethylene glycol) methyl ether only having an average molecular weight of 550 [17].
Three different samples with varying yields of DA were used: 77% of DA 1, 80% of DA 2, 70% of DA 3. The monomers were difficult to disperse in water; thus a maximum concentration of about 3 mM was used in this study. When the sample was irradiated, it changed colors from pale yellow to an intense blue. Samples were then printed out and placed on a hot plate to observe the color change which is displayed in Figure 4.
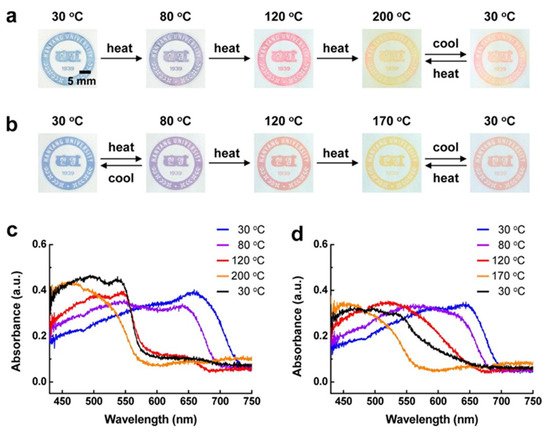
Figure 4. Photographs of printed images of (a) DA 2-derived PDAs and (b) DA 3-derived PDAs on an unmodified paper substrate upon thermal stimulation. UV–vis absorption spectra of (c) DA 2-derived PDAs and (d) DA 3-derived PDAs printed on an unmodified paper substrate as a function of the temperature. Black lines in each spectrum are the final phase involving cooling to 30 °C. Reproduced with the permission of Reference [17]. Copyright 2013 American Chemical Society.
Both DA2 and DA3 displayed shifts in their absorption spectra with change in temperature. In both samples, the color changed from blue to purple at 80 °C and then to red at 120 °C. At ~200 °C, the color turned yellowish red and, above that, it was yellow. Once cooled to 30 °C, the color changed to red. DA3 had two temperature ranges in which it displayed reversible color change (30 to 80 °C and 170 to 30 °C). This method had the potential to be applicable to paper-based devices, temperature sensors and even anti-counterfeiting systems [17].
PDA infused films have also been used for bacterial [18] and fungal detection [19] in biosensing applications and have been used for rapid bacterial detection in food [20,21]. While these are not thermochromic properties, they are still desirable qualities for food packaging and make PDA a fitting candidate for a possible multipurpose sensor.
Currently, there is a patent for a diacetylene thermochromic ink that has been used in food packaging. The invention is composed of a bi-block temperature-sensitive color-changing ink and exhibits uniform color component dispersion, strong viscosity with printed matter, and no color drop phenomenon [22].
1.2. Polyaniline (PANI)
There is a need for materials that are cost effective and durable while also having ease of workability and good accessibility when it comes to packaging. Polyaniline (PANI) is a promising candidate with all these features. It exhibits optimal chromogenic properties in a wide range of temperatures while maintaining its structural integrity [23].
PANI has been studied extensively for multiple purposes. In 2002, Rannou et al. showed that diester doped PANI exhibited two glass transition temperatures with a strong thermochromic effect in UV−vis−NIR spectra at sub-zero temperatures. 4-sulfophthalic acid was used as the dopant to protonate PANI and lower the glass transition temperature which improved the flexibility of the films created. It is assumed that the thermochromic phenomenon is induced by temperature dependent torsions within the polymer chain. These torsions cause a widening of the band gap within the now non-planar geometry of the polymer chain which results in a hypsochromic shift of the band corresponding to the π−π* transition [8].
PANI was protonated in solution with several diesters at 0.5 wt% and at a ratio that ensured full protonation of the doped polymer. Films were then made on polypropylene and glass substrates at 45 °C. Its molecular structure is shown in Figure 5. The thermochromic effect was characterized by recording solid-state UV−vis−NIR spectra of the films and the glass transition temperatures were determined by DSC measurements.
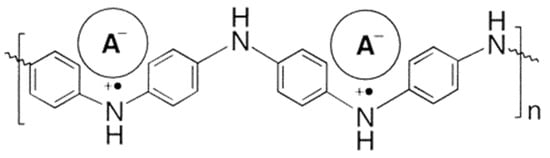
Figure 5. Molecular Structure of PANI(DEPSA)0.5. Reproduced with the permission of Reference [8]. Copyright 2002 American Chemical Society.
The study concluded that films made of PANI protonated with 1,2-(di-2-(butoxyethyl)) ester of 4-sulfophthalic acid (DBEEPSA) exhibited the strongest thermochromic effect at temperatures below the glass transition temperatures of the diester dopants. The UV-vis-NIR spectra of PANI(DBEEPSA)0.5 demonstrates that, above the glass transition temperature, Tg (−55.15 °C or 218 K), there are minimal changes in absorption spectra, but at 6.85 °C (280 K), there is a sharp peak at 440 nm and a notably broad absorption tail which can be attributed to delocalization of charge carriers in PANI [14]. Another small peak is observed at ~ 800 nm which is indicative of localized charge carriers present within the system. Similar spectral changes were observed in PANI doped with 1,2-(di-2-ethylhexyl) ester of 4-sulfophthalic acid (DEHEPSA) but at lower temperatures of T < -65.15 °C (208 K) which corresponds to the lower Tg of the dopant [8]. The optical spectra of these samples are presented in Figure 6.
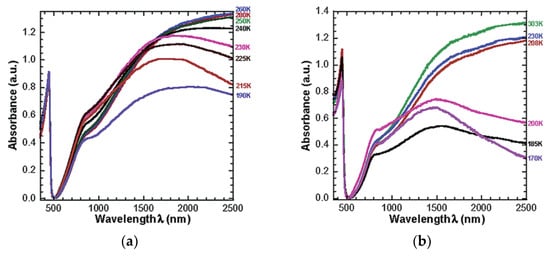
Figure 6. UV−vis−NIR spectra of (a) PANI(DBEEPSA)0.5 registered for the temperature range of 303−170 K (29.85 °C–103.15 °C). (b) PANI(DEHEPSA)0.5 registered for the temperature range of 280−190 K (6.85 °C–83.15 °C), Reproduced with the permission of Reference [8]. Copyright 2002 American Chemical Society.
The US Food and Drug Administration, US FDA, recommends that cold foods be transported at 40 °F or below which corresponds to ~4.45 °C (277.6 K) [24]. PANI(DBEEPSA)0.5 is the ideal candidate for food packaging as it exhibits thermochromic behavior at 6.85 °C (280 K).
Previously, PANI has also been shown to be an efficient scavenger of free radicals such as 1,1-diphenyl-2-picrylhydrazyl (DPPH). Free radicals are a known cause of food spoilage; thus, this property of PANI can be implemented in the protection of consumable goods. The antioxidant properties of conducting polymers were first evaluated by Ismail et al. by determining the efficacy of PANI films that were used to protect vulcanized rubber from oxidation and radiation deterioration [25]. Though other polymers have been reported to display similar properties, PANI’s superior free radical scavenging ability is unmatched [26].
In 2011, Nand investigated polyaniline for packaging applications by determining the free radical capacity of heat-treated samples of polyaniline of granular and micro/nanorod morphologies [27]. 600 mg specimens were heated for 30 min at temperatures ranging from 100 to 300 °C in steps of 15 °C and cooled to room temperature. A radical scavenging assay was performed by measuring the absorbance of a sample treated with DPPH using a spectrophotometer and the amount of remaining DPPH was calculated. It was observed that all morphologies of PANI exhibited free radical scavenging properties at elevated temperatures, but this ability declined after thermal treatment above 200 °C. The samples were amorphous at these temperatures. and thus, have the potential to be successfully merged with commonly used thermoplastics for food packaging applications [26].
Detailed studies have shown that PANI is also resistant to a broad range of bacteria including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, and Campylobacter jejuni [28,29,30,31]. PANI has been incorporated into surfaces or used as surface coatings for infection control and food safety applications [32]. It is thought that the inherent electrical conductivity of the polymer interferes with the negatively charged bacterial cell surface through electrostatic adherence [28,29,33]. Robertson and group found that PANI also stimulates hydrogen peroxide based oxidative stress in bacterial cells which causes damage and potential cell death [34]. This is significant because it can be incorporated into packaging to protect items from spoilage due to contamination from pathogenic agents.
This entry is adapted from the peer-reviewed paper 10.3390/mi12101193
This entry is offline, you can click here to edit this entry!
