Hypoxia has an important role in tumor progression via the upregulation of growth factors and cellular adaptation genes. These changes promote cell survival, proliferation, invasion, metastasis, angiogenesis, and energy metabolism in favor of cancer development. Hypoxia also plays a central role in determining the resistance of tumors to chemotherapy. Hypoxia of the tumor microenvironment provides an opportunity to develop new therapeutic strategies that may selectively induce apoptosis of the hypoxic cancer cells. Melatonin is well known for its role in the regulation of circadian rhythms and seasonal reproduction. Numerous studies have also documented the anti-cancer properties of melatonin including anti-proliferation, anti-angiogenesis, and apoptosis promotion. Here, we hypothesized that melatonin exerts the anti-cancer effects by inhibiting the hypoxia-induced pathways. Considering this action, co-administration of melatonin in combination with other therapeutic medications might increase the effectiveness of anti-cancer drugs. We explained the possible signaling pathways by which melatonin inhibits the hypoxia-induced cancer cell survival, invasion, migration, and metabolism as well as tumor angiogenesis (This context is extracted from our published review study (Bastani et al., 2021).
- melatonin
- cancer
- antioxidant
- apoptosis
- angiogenesis
- metastasis
1- Introduction: Hypoxia induces cancer (tumor) progression
Hypoxia occurs in many solid tumors and plays a role as a selective agent throughout metastatic transformation and progression (Terry et al., 2018). Although hypoxia negatively affects tumor proliferation in some conditions, it mainly allows tumor cells to adapt to insufficient oxygen and nutrients and consequently enhances the activity and aggressiveness of cancer cells. Moreover, genomic changes occurred in the tumor cells under low oxygen conditions can make it feasible for them to survive. In turn, the excessive proliferation of cancer cells exaggerates the hypoxic state. As a result, a vicious circle of hypoxia and tumor progression develops (Vaupel, 2004). Hypoxia is also associated with genomic instability and induces malignant phenotypes such as apoptosis resistance (Vaupel et al., 2001). Furthermore, poor vascularity reduces tumor cell exposure to the drugs during chemotherapy and oxidative damage during radiotherapy; thus, it is common for tumors to develop resistance to chemotherapy and radiotherapy under hypoxic conditions (Vaupel, 2004).
Hypoxia up-regulates growth factors and cellular adaptation genes by increasing the levels of HIF proteins which have a significant impact on cancer progression (Marin-Hernandez et al., 2009). Interestingly, mutations that cause either oncogene activation or tumor suppressor inactivation can increase HIF-1α expression in cancer cells (Bristow and Hill, 2008). Hypoxia positively induces survival, invasion, migration, metabolism, and angiogenesis in cancer cells as summarized in Figure 1.
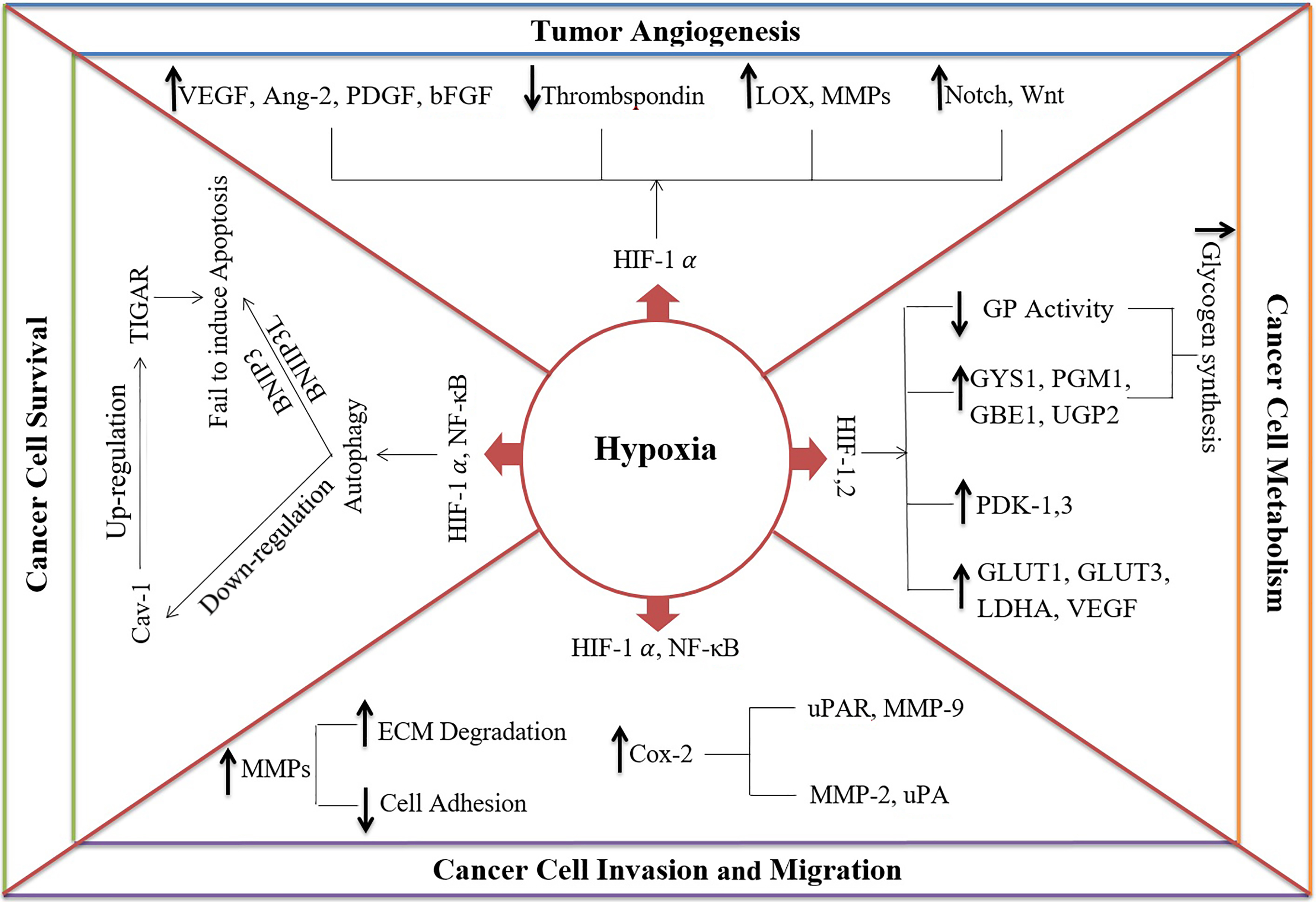
Figure 1. The mechanisms of hypoxia in cancer progression:
- Hypoxia enhances cancer cell survival by inducing autophagy via hypoxia-inducible factor-1 alpha (HIF-1α) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF- κB). Subsequently, it causes 1) down-regulation of caveolin-1 (Cav-1), leading to up-regulation of TP53-inducible glycolysis and apoptosis regulator (TIGAR) and protecting cells against oxidative stress and apoptosis and 2) Protecting the cells from apoptosis via BCL2 Interacting Protein 3 (BNIP3) and BCL2 Interacting Protein 3 Like (BNIP3L).
- Hypoxia can induce tumor angiogenesis by 1) increasing proangiogenic factors such as vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), 2) decreasing angiogenesis inhibitors like thrombospondin, 3) upregulation of extracellular matrix (ECM) proteins, such as lysyl oxidase (LOX) and matrix metalloproteinases (MMPs), and 4) activating Notch and Wnt signaling pathways.
- Hypoxia increases cancer cell invasion and migration by down-regulation of cell adhesion molecules and up-regulation of ECM degradation molecules such as MMP-9 and Urokinase-type plasminogen activator receptor (uPAR)
- Hypoxia affects the metabolic pathways to provide high energy for cancer cells by 1) enhancing transcription of glucose transporters genes (GLUT1 and GLUT3), VEGF, and glycolytic enzymes (e.g. lactate dehydrogenase, LDHA), 2) inducing expression of glycogenesis enzymes including phosphoglucomutase-1 (PGM1), glycogen synthase-1 (GYS1), UDP-Glucose Pyrophosphorylase 2 (UGP2) and 1,4-alpha-glucan branching enzyme 1 (GBE1), 3) reducing Glycogen phosphorylase (GP) activity, and 4) diverting pyruvate from the citric acid cycle into lactate by pyruvate dehydrogenase kinases-1 and -3 (PDK-1 and -3)
2- Melatonin as a proposed therapeutic factor for inhibition of hypoxia-induced tumor progression
As discussed in previous section, hypoxia is an important factor in tumor progression that positively affects survival, angiogenesis, invasion, migration, and the metabolic status of cancer cells. Hypoxia also contributes to the radioresistance and chemoresistance of the tumor. Melatonin is a potent anti-tumor agent that likely inhibits various hypoxia-induced signaling pathways in cancer cells. Thus, we propose that a way by which melatonin inhibits cancer growth and progression and also improves therapeutic efficacy is the inhibition of hypoxia-induced survival, angiogenesis, migration, and invasion. The Figure 2 describes how melatonin prevents hypoxia-induced properties of cancer cells.
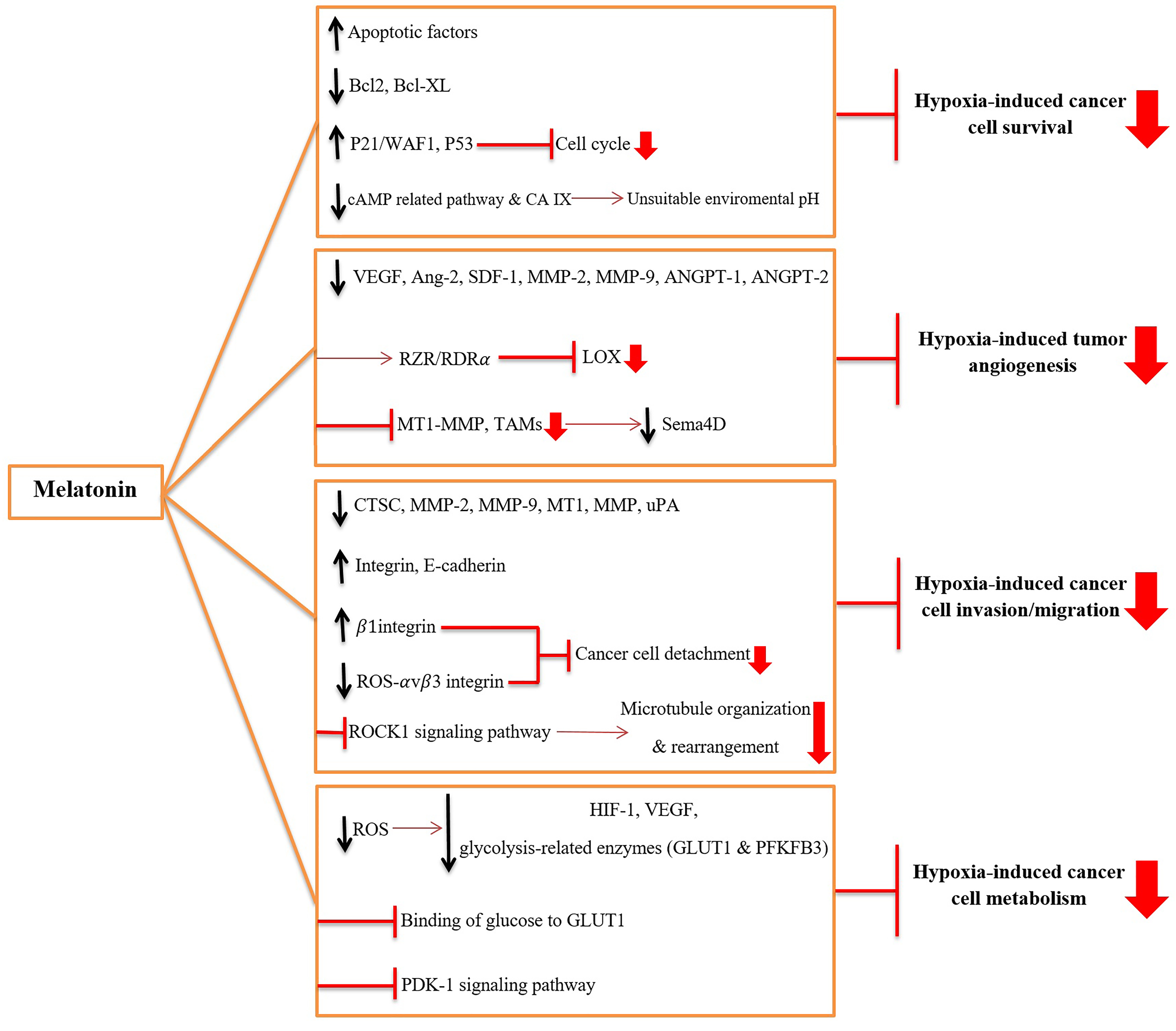
Figure 2. The mechanisms through which melatonin inhibits hypoxia-induced tumor progression:
- Melatonin inhibits survival of hypoxic cancer cells by 1) up-regulating and activating the apoptotic factors, 2) down-regulating and inactivating anti-apoptotic factors [B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xL)], 3) blocking cell cycle by up-regulating p21/WAF1 and p53, and 4) inhibiting carbonic anhydrase IX (CA IX) expression and activity and cAMP-related pathways to make an unsuitable environmental pH
- Melatonin inhibits hypoxia-induced angiogenesis by 1) suppressing the activity of vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2), stromal-derived factor 1 (SDF-1), matrix metalloproteinase-2 and -9 (MMP-2 and -9), angiopoietin-1 and -2 (ANGPT-1 and -2), 2) inhibiting the expression of lipoxygenase (LOX) via interacting with RZR/RORα nuclear receptor, and 3) blocking the hypoxia-induced tumor-associated macrophages (TAMs) and membrane-type 1 matrix metalloproteinase (MT1-MMP) activity and subsequently reducing Semaphorin-4D (Sema4D).
- Melatonin inhibits hypoxia-induced invasion and migration of cancer cells by 1) decreasing levels of proteases including Cathepsin C (CTSC), MMP-2, MMP-9, MT1-MMP, and urokinase-type plasminogen activator (uPA), 2) up-regulating the adhesion proteins, such as integrin and E-cadherin, 3) suppressing oxidative stress-induced detachment of cancer cells via overexpression of the 1 integrin and down-regulation of ROS-αvβ3 integrin-FAK/Pyk2 (Focal adhesion kinase/ proline-rich tyrosine kinase 2) signaling pathway, and 4) blocking hypoxia-induced microtubule organization and rearrangement via blocking Rho-kinase 1 (ROCK1) signaling pathway.
- Melatonin disturbs hypoxia-induced cancer cell metabolism by 1) reducing reactive oxygen species (ROS) and down-regulating hypoxia-Inducible Factor-1 (HIF-1), VEGF and glycolysis-related enzymes such as glucose transporter 1 (GLUT1) and progestins activate 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), and 2) competing with glucose in binding to GLUT1, and 3) inhibition of 3-phosphoinositide-dependent protein kinase 1 (PDK-1) signaling pathway.
3- Conclusion
It is well-documented that hypoxia is involved in tumor progression via various mechanisms, including induction of cancer cell invasion and migration, tumor angiogenesis, and modification of cell metabolism. On the contrary, melatonin can act as an anti-tumor agent partly through the inhibition of hypoxia-induced pathways. Herein, we discussed the possible signaling pathways by which melatonin inhibits hypoxia-induced cancer cell survival, invasion, migration, metabolism as well as tumor angiogenesis. The accumulated data overwhelmingly support the idea that melatonin is an anti-cancer agent independently or in combination with other chemotherapeutic agents. Considering melatonin efficacy and safety, it should be considered as part of the therapeutic regimen to treat certain types of cancer. Additional studies would further clarify the mechanisms by which melatonin acts as an oncostatic agent including the details of the proposed outline in this report.
References
Bastani, S., Akbarzadeh, M., Rastgar Rezaei, Y., Farzane, A., Nouri, M., Mollapour Sisakht, M., Fattahi, A., Akbarzadeh, M., Reiter, R.J. 2021. Melatonin as a Therapeutic Agent for the Inhibition of Hypoxia-Induced Tumor Progression: A Description of Possible Mechanisms Involved. International journal of molecular sciences, 22, 10874-10888.
Terry, S., Faouzi Zaarour, R., Hassan Venkatesh, G., Francis, A., El-Sayed, W., Buart, S., Bravo, P., Thiery, J. & Chouaib, S. 2018. Role of hypoxic stress in regulating tumor immunogenicity, resistance and plasticity. International journal of molecular sciences, 19, 3044.
Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. 2009. HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini reviews in medicinal chemistry, 9, 1084-1101.
Bristow, R. G. & Hill, R. P. 2008. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nature Reviews Cancer, 8.
Vaupel, P. 2004. The role of hypoxia‐induced factors in tumor progression. The oncologist, 9, 10-17.
Vaupel, P., Thews, O. & Hoeckel, M. 2001. Treatment resistance of solid tumors. Medical oncology, 18, 243-259.
This entry is adapted from the peer-reviewed paper 10.3390/ijms221910874
