Cirrhotic cardiomyopathy (CCM), cardiac dysfunction in end-stage liver disease in the absence of prior heart disease, is an important clinical entity that contributes significantly to morbidity and mortality. The original definition for CCM, established in 2005 at the World Congress of Gastroenterology (WCG), was based upon known echocardiographic parameters to identify subclinical cardiac dysfunction in the absence of overt structural abnormalities. Subsequent advances in cardiovascular imaging and in particular myocardial deformation imaging have rendered the WCG criteria outdated. A number of investigations have explored other factors relevant to CCM, including serum markers, electrocardiography, and magnetic resonance imaging. CCM characteristics include a hyperdynamic circulatory state, impaired contractility, altered diastolic relaxation, and electrophysiological abnormalities, particularly QT interval prolongation. It is now known that cardiac dysfunction worsens with the progression of cirrhosis. Treatment for CCM has traditionally been limited to supportive efforts, but new pharmacological studies appear promising. Left ventricular diastolic dysfunction in CCM can be improved by targeted heart rate reduction. Ivabradine combined with carvedilol improves left ventricular diastolic dysfunction through targeted heart rate reduction, and this regimen can improve survival in patients with cirrhosis. Orthotopic liver transplantation also appears to improve CCM.
- cirrhosis
- cardiomyopathy
- echocardiography
- treatment
- diagnosis
- pathophysiology
- Ejection Fraction
- Diastolic Heart Failure
- Cardiac MRI
- Speckled Tracking Echocardiography
1. Introduction
Cirrhosis is associated with multiple cardiovascular changes including the development of a hyperdynamic circulation and cardiac dysfunction. Cirrhotic cardiomyopathy, a form of cardiac failure associated with cirrhosis of any cause, is the end result of complex circulatory and humoral changes. Cirrhotic cardiomyopathy may have a prevalence of up to 60% [1]. It is present in both adult and paediatric patients with cirrhosis and a major cause for morbidity and mortality [2]. The Gastroenterology World Congress 2005 defined a triad (Table 1) of clinical features associated with cirrhotic cardiomyopathy, such as systolic dysfunction, seen as a blunting of the usual cardiac response to stress; diastolic dysfunction; and electrical dysfunction [3][4]. The technical definition [5] (Table 1) has not kept pace with recent advances in cardiac imaging and the diagnosis of systolic and diastolic dysfunction. Izzy et al. have proposed new technical definitions [6] (Table 2) based on recent advances in cardiac imaging. In this review, we will comprehensively outline up to date research on the pathophysiology of CCM and address the latest advances in diagnostic imaging and management.
Table 1. Diagnostic criteria for cirrhotic cardiomyopathy, proposed by the Gastroenterology World Congress.
| Systolic Dysfunction | Diastolic Dysfunction | Supportive Criteria |
|---|---|---|
| Blunted increase in cardiac output on exercise, volume challenge or pharmacological stimuli | E/A ratio <1.0 (age-corrected) | Prolonged Q-Tc interval |
| Resting ejection fraction <55% | Prolonged deceleration time (<200 ms) | Enlarged left atrium |
| Prolonged isovolumetric relaxation time (<80 ms) | Increased myocardial mass | |
| Electrophysiological abnormalities | Increased BNP and pro-BNP | |
| Abnormal chronotropic response | Increased troponin I | |
| Electromechanical uncoupling/dys-synchrony |
Reported by Møller et al. [5].
Table 2. Proposed updated criteria for cirrhotic cardiomyopathy.
| Systolic Dysfunction | Advanced Diastolic Dysfunction | Areas for Future Research Which Require Further Validation |
|---|---|---|
| Any of the following LV ejection fraction ≤50 % | ≥3 of the following | Abnormal chronotropic or inotropic response |
| Absolute GLS <18% or >22% | Septal é velocity <7 cm/s | Electrocardiographic changes |
| E/é ratio ≥15 | Electromechanical uncoupling | |
| LAVI >34 mL/m2 | Myocardial mass change | |
| TR velocity >2.8 m/s | Serum biomarkers | |
| Chamber enlargement | ||
| CMRI |
Modified from Izzy et al. [6].
2. Pathophysiology
Cirrhosis and portal hypertension lead to a hyperdynamic circulatory state, defined by an increase in total blood volume, a reduction in circulating volume due to splanchnic blood pooling, reduced systemic vascular resistance, and an increased cardiac output. In addition to these circulatory changes, there is direct injury to cardiac myocytes due to a combination of pro-inflammatory cytokines, elaboration of vasoactive peptides, and reduced response to sympathetic stimulation. A rat cirrhotic model has revealed that alterations in collagen configuration and titin modulation can lead to changes in diastolic function. [7]
Early dynamic changes are largely driven by humoral responses. Splanchnic arterial vasodilation and a reduced hepatic capacity to metabolize vasoactive agents leads to a reduced effective arterial circulating volume and the activation of the central baroreceptors, leading to the activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) (see Figure 1) [4]. This leads to the development of a hyperdynamic circulation, which is defined by an increased cardiac output, reduced arterial blood pressure, reduced vascular resistance, and increased heart rate [8].
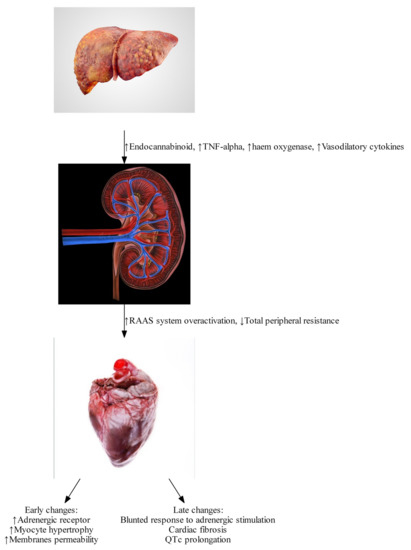
Figure 1. Pathophysiological changes found in Cirrhotic Cardiomyopathy. (Image courtesy: iStock photo: https://www.istockphoto.com/photos/) (All images have creative license).
Late dynamic changes result from macroscopic anatomical alteration in cardiac structure and histopathological changes in myocytes.
2.1. Endocannabinoids
Endocannabinoids such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) bind to the cannabinoid-1 (CB-1) receptor. The endocannabinoid system is significantly up-regulated in the setting of cirrhotic cardiomyopathy [9][10] due to increased liver production of endocannabinoids, as well as increased expression of cannabinoid receptors. Activation of the endocannabinoid system can lead to reduced peripheral vascular resistance and increased splanchnic blood volume, worsening the effects of portal hypertension [11][12]. In animal models, improvements in peripheral vascular resistance, arterial blood pressure, and in cardiac β adrenergic response have been seen after treatment with cannabinoid receptor antagonists [11].
2.2. Tumor Necrosis Factor
Tumor necrosis factor (TNF)-α has been shown to induce the formation of endocannabinoids [10][12], and to promote a pro-inflammatory state, which further depresses cardiac function via activation of inducible nitric oxide synthase and elaboration of nitric oxide [13]. Nitric oxide has a direct negative inotropic effect, causing a cardio-depression similar to sepsis. This effect contributes to the early development of hyperdynamic circulation resulting from endeavors to compensate through other mechanisms. In a bile duct ligated mouse model, use of inducible nitric oxide inhibitors has been shown to reverse cardiac depression [13].
2.3. Haem Oxygenase
Haem oxygenase catalyzes the oxidation of haemoglobin into carbon monoxide and other products [14]. Carbon monoxide is thought to be commensurable with nitric oxide in biological systems in regulation and production, and, correspondingly, is thought to have a role as a secondary messenger [14].
Haem oxygenase has been shown to be up-regulated in cirrhotic rats [15], and to correlate with higher cardiac haem oxygenase levels. Haem oxygenase mRNA expression increases with worsening Child-Pugh status [16]. Exhaled carbon monoxide is also increased in patients with cirrhosis. In vitro research has demonstrated that the impaired cardiac contractility in a bile duct ligated mouse model can be reversed by regulating the haem oxygenase-carbon monoxide system [15].
2.4. Bile Acid
Animal models have demonstrated the negative inotropic effect of elevated bile acid levels [17]. Elevated bile acid levels cause cardiac arrhythmia and disruption of channels, leading to cardiotoxicity [17][18] Elevated bile acids are thought to have cardiotoxic effects in human hearts, although ursodeoxycholic acid has been shown to have a cardioprotective effect [19]. There is a need for more in vitro studies before the effects of bile acids on human cardiac system can be fully comprehended.
2.5. β Adrenergic Responsiveness
β receptors in cardiac tissue respond to noradrenalin (norepinephrine) released from sympathetic innervation, resulting in an increased heart rate (positive chronotropy), and an increase in cardiac contractility (positive inotropy). Chronic activation of the sympathetic nervous system in cirrhotic cardiomyopathy due to activation of systemic baroreceptors leads to constant stimulation of the cardiomyocytes with noradrenalin. Results in animal models have demonstrated reduced β receptor expression in this setting [20], resulting in an inability to raise cardiac chronotropy and inotropy, and blunted cardiac responsiveness to stress [21].
2.6. Myocardial Fibrosis and Myocyte Hypertrophy
Prolonged exposure to β-adrenergic stimulation and an adverse biochemistry profile inadvertently leads to the remodeling of myocytes. In contemporary settings, this has been remarkably absent, possibly due to advances in the early recognition of cirrhotic patients, and to medical attempts to correct the biochemistry and reverse potential anatomical pathophysiology. Historical studies done in the 1950s by Lunseth et al. [22] showed features of ventricular hypertrophy in patients with liver cirrhosis. Hearts from patients with cirrhosis weighed much more than standard populations at that time. Given diagnostic criteria at that time, those patients would meet criteria for CCM (Cirrhotic cardiomyopathy). Further histopathology showed remarkable features of ventricular hypertrophy, with larger nuclear vacuole and increased cytoplasmic depositing of fibrin (see Figure 2). This study also showed a quantitative relationship between the degree of disease severity and degree of cardiac hypertrophy. In twelve cases in these studies, there were features of cardiac fibrosis, with features of fibrous tissues, and these were associated with a longer duration of liver disease [22]. Studies done by Jellis et al. [23] also show similar features of cardiac fibrosis in late-stage liver cirrhosis. In addition to that, the latest contemporary study performed by Wiese et al. [24] shows features of increased fibrogenesis and increased extracellular volume in myocardium in cirrhotic, both with biochemical and anatomical evidence, further substantiating proof that cirrhosis does lead to permanent scarring of the myocardium through an inflammatory process.
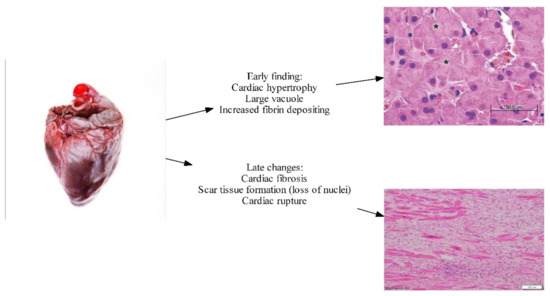
Figure 2. Early and late histopathological changes in CCM (Image courtesy of Marian and Braunwald, 2017) [25].
2.7. Altered Cardiomyocyte Membrane Fluidity and Ion Channel Defects
Ion flux, particularly calcium (Ca2+), potassium (K+), and sodium (Na+), plays a substantial role in effective contractility. Calcium influx is required to maintain the plateau of the cardiac action potential and to allow for the uncovering of binding sites along the myosin filament. Calcium influx is also critical in calcium-induced calcium release, whereby a small amount of extra-cellular calcium influx is magnified by calcium release from the endoplasmic reticulum.
Potassium and sodium are both required for the generation of cardiac action potentials. Electrocardiograph changes are common in patients with cirrhosis. Corrected QT (QTc) intervals have been shown to correlate with the degree of cirrhosis [26], and up to 60 percent of patients with cirrhosis have a prolonged QTc interval [27].
Results from animal models of cirrhotic cardiomyopathy have shown a diminished capacity for ion transport across the cell membrane [28][29]. These changes contribute to both impaired cardiac contractility and electrophysiological abnormalities [21], including prolonged QTc. An increase in membrane cholesterol content has been associated with these changes, although the underlying mechanism is unclear.
3. Treatment of CCM
Studies on CCM treatment are extremely limited. This may be largely because CCM was recognized as a temporary phenomenon that could be corrected with a definite treatment plan of liver transplantation [30]. However, studies emerging within recent decades indicate a paradigm shift as CCM is being recognized as a major co-morbidity that requires expert evaluation with specific treatment end goals that must to be met to improve morbidity and mortality outcomes [31].
Treatment for cirrhotic cardiomyopathy is an area of ongoing research and includes a number of challenges. Patients recruited to clinical trials tend to have less severe cirrhosis [32]; however, most patients diagnosed with cirrhotic cardiomyopathy will have advanced cirrhosis. This creates a dilemma of whether treatment outcomes could be extrapolated and applied to other patients with CCM. Despite this challenge, investigation into the basic mechanisms of cirrhotic cardiomyopathy has uncovered some potential treatment targets, including the pro-inflammatory system and the endocannabinoid system [33]. Other treatments under investigation include traditional heart failure medications, with some trials showing positive results [34].
3.1. β Blockade
β blockers are the standard of care for many forms of cardiac failure, and their use improves mortality in heart failure with reduced ejection fraction [35]. However, cirrhosis is often an exclusion criterion in these trials. In patients with cirrhosis, β-blockers have been studied in the treatment of portal hypertension [36]. Randomized controlled trials of metoprolol and carvedilol in patients with cirrhotic cardiomyopathy showed no significant differences in mortality, hospitalizations, or cardiac function [30]. It was postulated that negative results may have been due to inadequate heart rate suppression in these patients [30].
A trial performed by Silvestri et al. [30] managed to suppress the heart rate to less than 65 beats per minute, which is the standard for other clinical trials in cardiology. Despite this, the trial failed to show any differences in mortality, hospitalizations, or cardiac function [30]. This trial was limited to a small sample size, and lacked variation in β blockers being tested, limiting the possible inferences and increasing the likelihood of type II errors [30].
3.2. Ivabradine
Ivabradine, the Inward sodium potassium channel within sinoatrial node (If, or ‘funny channel’) inhibitor, has been shown to improve mortality in combination with beta blockers when used in patients with reduced ejection fraction [37]. The combination of ivabradine and carvedilol improves mortality in patients with cirrhotic cardiomyopathy with diastolic dysfunction compared with standard care [34]. This was demonstrated by Premkumar et al. [34] in a trial where a combination of low dose ivabradine and carvedilol were compared head-to-head with standard cirrhotic liver care. There was an improvement in mortality and a reduction in liver cirrhosis-related events in patients who were able to achieve a heart rate of 55 to 65 beats per minute.
Despite this positive outcome, it is important to recognize that these are early studies of the application of anti-congestive cardiac failure medication in CCM patients. The dose utilized by the Premkumar et al. [34] trial was low, with none of the known potential side effects, such as atrial fibrillation due to ivabradine, observed in patients in the treatment arm. One interpretation of these findings is that the boundary was perhaps not pushed hard enough out of an overabundance of caution. It will be critical to conduct more extensive studies, particularly on dosing, before β blockers and ivabradine become more routinely used in CCM.
3.3. Liver Transplantation
Patients with cirrhotic cardiomyopathy face significant risk in the pre-operative, intraoperative, and peri-operative period [38]. Liver transplantation has been thought of as the definitive intervention for cirrhotic cardiomyopathy [4][39]. Self-control studies have shown a significant reversal of cardiac changes post-transplant [38]. There is hope that the underlying process leading to improvement can be elucidated, and lead to future targeted therapies [4]. Following transplantation, there are improvements in QTc, exercise tolerance, echocardiography parameters, and biochemistry such as troponin and BNP. A summary of the issues for this discussion is shown in Figure 3.

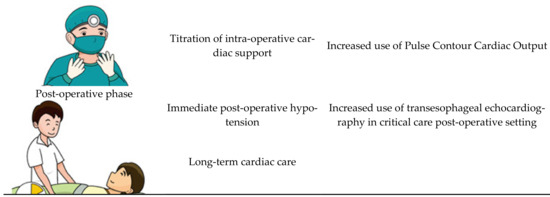
Figure 3. The peri-transplant setting.
In the pre-transplantation period, patients with CCM have a high prevalence of atrial fibrillation and QTc prolongation, along with cardiac failure-related complications including biventricular failure, pulmonary hypertension and cardio-renal diseases.
While it remains true that transplantation will reverse the majority of the pathology, some pathological processes may remain active beyond the peri-transplant period. For that reason, it is now routine practice for the screening of CCM to be done in all liver transplant candidates. Screening methods involve traditional transthoracic echocardiography or stress echocardiography. The application of transthoracic echocardiography in the paediatric population has been shown to significantly improve care by identifying high risk patients prior to transplantation [40]. Less favorable transthoracic echocardiography parameters are associated with a prolonged ICU length of stay in the post-transplant period, making it an invaluable pre-operative assessment tool [41]. We suggest that cardiac MRI should also be routinely performed where possible, given that it provides valuable information regarding cardiac function as well as for risk-stratifying the patient [42].
During the intra-operative state, patients with CCM have a much higher adverse outcome due to complex fluid status as well as significant negative inotropic effects of general anesthesia on a cardiovascular system already under stress [43]. Most transplant cases last for hours, and data is emerging which shows that, for patients with CCM, invasive blood pressure monitoring does not truly reflect perfusion [44]. There is thus a growing consensus that transesophageal echocardiography should be performed to help titrate inotropic support intra-operatively [45]. The application of echocardiography intra-operatively, however, is currently limited to specialized institutions, given that not all anaesthetists are trained in evaluating the cardiac output on invasive echocardiography. There is emerging evidence that comprehensive cardiac output assessment, such as pulse contour cardiac output, should be standard of care in all liver transplantations [43].
During the post-operative period, there are major changes in vascular resistance along with portal venous system that generate major stresses on the cardiovascular system [43]. These patients already face a challenging transplantation journey from a significant pre-transplant disease burden [46]. Unsurprisingly, a significant number of cardiac complications has been observed in transplant patients, with the most common being refractory hypotension [47]. The current literature suggests that transesophageal echocardiography should be done immediately post-transplant as standard of care, because it provides invaluable information relevant to the required level of post-transplant cardiac support [41] (see Figure 3).
This entry is adapted from the peer-reviewed paper 10.3390/gastroent12010008
References
- Razpotnik, M.; Bota, S.; Wimmer, P.; Hackl, M.; Lesnik, G.; Alber, H.; Peck-Radosavljevic, M. The prevalence of cirrhotic cardiomyopathy according to different diagnostic criteria. Liver Int. 2020.
- Desai, M.S.; Penny, D.J. Bile acids induce arrhythmias: Old metabolite, new tricks. Heart 2013, 99, 1629–1630.
- Hamoudi, W.A.; Lee, S.S. Cirrhotic cardiomyopathy. Ann. Hepatol. 2006, 5, 132–139.
- Møller, S.; Danielsen, K.V.; Wiese, S.; Hove, J.D.; Bendtsen, F. An update on cirrhotic cardiomyopathy. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 497–505.
- Møller, S.; Henriksen, J.H. Cardiovascular complications of cirrhosis. Gut 2008, 57, 268–278.
- Izzy, M.; VanWagner, L.B.; Lin, G.; Altieri, M.; Findlay, J.Y.; Oh, J.K.; Watt, K.D.; Lee, S.S. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology 2020, 71, 334–345.
- Glenn, T.K.; Honar, H.; Liu, H.; ter Keurs, H.E.; Lee, S.S. Role of cardiac myofilament proteins titin and collagen in the pathogenesis of diastolic dysfunction in cirrhotic rats. J. Hepatol. 2011, 55, 1249–1255.
- Møller, S.; Bendtsen, F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018, 38, 570–580.
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid signaling and liver therapeutics. J. Hepatol. 2013, 59, 891–896.
- Yang, Y.-Y.; Liu, H.; Nam, S.W.; Kunos, G.; Lee, S.S. Mechanisms of tnfα-induced cardiac dysfunction in cholestatic bile duct-ligated mice: Interaction between tnfα and endocannabinoids. J. Hepatol. 2010, 53, 298–306.
- Caraceni, P.; Domenicali, M.; Giannone, F.; Bernardi, M. The role of the endocannabinoid system in liver diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 65–77.
- Toshikuni, N.; Ozaki, K.; George, J.; Tsutsumi, M. Serum endocan as a survival predictor for patients with liver cirrhosis. Can. J. Gastroenterol. Hepatol. 2015, 29, 427–430.
- Liu, H.; Ma, Z.; Lee, S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology 2000, 118, 937–944.
- Maines, M.D. The heme oxygenase system:A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554.
- Liu, H.; Song, D.; Lee, S.S. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G68–G74.
- Bessa, S.S.E.-D.; Ali, E.M.M.; El-Wahab, A.E.-S.A.; El-Din, S.A.E.-M.N. Heme oxygenase-1 mrna expression in egyptian patients with chronic liver disease. Hepat. Mon. 2012, 12, 278–285.
- Gazawi, H.; Ljubuncic, P.; Cogan, U.; Hochgraff, E.; Ben-Shachar, D.; Bomzon, A. The effects of bile acids on beta-adrenoceptors, fluidity, and the extent of lipid peroxidation in rat cardiac membranes. Biochem. Pharmacol. 2000, 59, 1623–1628.
- Desai, M.S.; Zainuer, S.; Kennedy, C.; Kearney, D.; Goss, J.; Karpen, S.J. Cardiac structural and functional alterations in infants and children with biliary atresia, listed for liver transplantation. Gastroenterology 2011, 141, 1264–1272.e1–4.
- Rainer, P.P.; Primessnig, U.; Harenkamp, S.; Doleschal, B.; Wallner, M.; Fauler, G.; Stojakovic, T.; Wachter, R.; Yates, A.; Groschner, K.; et al. Bile acids induce arrhythmias in human atrial myocardium--implications for altered serum bile acid composition in patients with atrial fibrillation. Heart 2013, 99, 1685–1692.
- Lee, S.S.; Marty, J.; Mantz, J.; Samain, E.; Braillon, A.; Lebrec, D. Desensitization of myocardial β-adrenergic receptors in cirrhotic rats. Hepatology 1990, 12, 481–485.
- Wiese, S.; Hove, J.D.; Bendtsen, F.; Møller, S. Cirrhotic cardiomyopathy: Pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2013, 11, 177–186.
- Lunseth, J.H.; Olmstead, E.G.; Forks, G.; Abboud, F. A study of heart disease in one hundred eight hospitalized patients dying with portal cirrhosis. Arch. Intern. Med. 1958, 102, 405–413.
- Jellis, C.L.; Kwon, D.H. Myocardial t1 mapping: Modalities and clinical applications. Cardiovasc. Diagn. Ther. 2014, 4, 126–137.
- Wiese, S.; Voiosu, A.; Hove, J.D.; Danielsen, K.V.; Voiosu, T.; Grønbaek, H.; Møller, H.J.; Genovese, F.; Reese-Petersen, A.L.; Mookerjee, R.P.; et al. Fibrogenesis and inflammation contribute to the pathogenesis of cirrhotic cardiomyopathy. Aliment. Pharmacol. Ther. 2020, 52, 340–350.
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770.
- Bernardi, M.; Calandra, S.; Colantoni, A.; Trevisani, F.; Raimondo, M.L.; Sica, G.; Schepis, F.; Mandini, M.; Simoni, P.; Contin, M.; et al. Q-t interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998, 27, 28–34.
- Ruíz-del-Árbol, L.; Serradilla, R. Cirrhotic cardiomyopathy. World J. Gastroenterol. 2015, 21, 11502.
- Ward, C.A.; Liu, H.; Lee, S.S. Altered cellular calcium regulatory systems in a rat model of cirrhotic cardiomyopathy. Gastroenterology 2001, 121, 1209–1218.
- Jaue, D.; Ma, Z.; Lee, S. Cardiac muscarinic receptor function in rats with cirrhotic cardiomyopathy. Hepatology 1997, 25, 1361–1365.
- Silvestre, O.M.; Farias, A.Q.; Ramos, D.S.; Furtado, M.S.; Rodrigues, A.C.; Ximenes, R.O.; de Campos Mazo, D.F.; Yoshimura Zitelli, P.M.; Diniz, M.A.; Andrade, J.L.; et al. β-blocker therapy for cirrhotic cardiomyopathy: A randomized-controlled trial. Eur. J. Gastroenterol. Hepatol. 2018, 30, 930–937.
- Zardi, E.M.; Zardi, D.M.; Chin, D.; Sonnino, C.; Dobrina, A.; Abbate, A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J. Cardiol. 2016, 67, 125–130.
- Buggey, J.; Alenezi, F.; Yoon, H.J.; Phelan, M.; DeVore, A.D.; Khouri, M.G.; Schulte, P.J.; Velazquez, E.J. Left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: Outcomes following an acute heart failure hospitalization. ESC Heart Fail. 2017, 4, 432–439.
- Nanashima, A.; Pillay, P.; Crawford, M.; Nakasuji, M.; Verran, D.J.; Painter, D. Analysis of postrevascularization syndrome after orthotopic liver transplantation: The experience of an Australian liver transplantation center. J. Hepatobiliary Pancreat. Surg. 2001, 8, 557–563.
- Premkumar, M.; Rangegowda, D.; Vyas, T.; Khumuckham, J.S.; Shasthry, S.M.; Thomas, S.S.; Goyal, R.; Kumar, G.; Sarin, S.K. Carvedilol combined with ivabradine improves left ventricular diastolic dysfunction, clinical progression, and survival in cirrhosis. J. Clin. Gastroenterol. 2019, 54, 561–568.
- Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). Lancet 1999, 353, 2001–2007.
- Timoh, T.; Protano, M.; Wagman, G.; Bloom, M.; Vittorio, T. A perspective on cirrhotic cardiomyopathy. Transplant. Proc. 2011, 43, 1649–1653.
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (shift): A randomised placebo-controlled study. Lancet 2010, 376, 875–885.
- Liu, H.; Jayakumar, S.; Traboulsi, M.; Lee, S.S. Cirrhotic cardiomyopathy: Implications for liver transplantation. Liver Transplant. 2017, 23, 826–835.
- Carvalho, M.; Kroll, P.; Kroll, R.; Carvalho, V. Cirrhotic cardiomyopathy: The liver affects the heart. Braz. J. Med. Biol. Res. 2019, 52.
- Gorgis, N.M.; Kennedy, C.; Lam, F.; Thompson, K.; Coss-Bu, J.; Akcan Arikan, A.; Nguyen, T.; Hosek, K.; Miloh, T.; Karpen, S.J.; et al. Clinical Consequences of Cardiomyopathy in Children With Biliary Atresia Requiring Liver Transplantation. Hepatology 2019, 69, 1206–1218.
- Junge, N.; Junge, C.; Schröder, J.; Pfister, E.D.; Leiskau, C.; Hohmann, D.; Beerbaum, P.; Baumann, U. Pediatric cirrhotic cardiomyopathy: Impact on liver transplant outcomes. Liver Transpl. 2018, 24, 820–830.
- Isaak, A.; Praktiknjo, M.; Jansen, C.; Faron, A.; Sprinkart, A.M.; Pieper, C.C.; Chang, J.; Fimmers, R.; Meyer, C.; Dabir, D.; et al. Myocardial fibrosis and inflammation in liver cirrhosis: Mri study of the liver-heart axis. Radiology 2020, 297, 51–61.
- Pietri, L.D.; Mocchegiani, F.; Leuzzi, C.; Montalti, R.; Vivarelli, M.; Agnoletti, V. Transoesophageal echocardiography during liver transplantation. World J. Hepatol. 2015, 7, 2432.
- Della Rocca, G.; Costa, M.G.; Pompei, L.; Chiarandini, P. The liver transplant recipient with cardiac disease. Transplant. Proc. 2008, 40, 1172–1174.
- Oxorn, D.C. Intraoperative echocardiography. Heart 2008, 94, 1236–1243.
- Gaskari, S.A.; Honar, H.; Lee, S.S. Therapy insight: Cirrhotic cardiomyopathy. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 329–337.
- Voiosu, A.M.; Bălănescu, P.; Daha, I.; Smarandache, B.; Rădoi, A.; Mateescu, R.B.; Băicuş, C.R.; Voiosu, T.A. The diagnostic and prognostic value of serum endocan in patients with cirrhotic cardiomyopathy. Rom. J. Intern. Med. 2018, 56, 182–192.
