Curcumin, a naturally occurring polyphenol, has been recently proposed for the management of neurodegeneration. It is possible that curcumin could exert direct regulative effects primarily in the gastrointestinal tract, where high concentrations of curcumin are present after oral administration.
- curcumin
- gut microbioma
- polyphenols
- bioactivity
- metabolism
- neuroprotection
1. Introduction
Over the last decades, the interest in herbal medicines has greatly increased since they can exert preventive effects against chronic and degenerative diseases including cancer. In addition, these molecules play an important role in neuroprotection by modulating different cellular functions. Different studies have shown that curcumin, like others dietary polyphenols, is able to counteract the effects of toxic damage in different tissues [1][2].Polyphenols are a large class of compounds produced from plants as secondary metabolites. Contrasting findings on the bioavailability of polyphenols created doubts about their usefulness as beneficial antioxidant compounds. Currently, there are findings showing that polyphenols can exert their biological effects following chemical modifications performed by gut microbiota. Indeed, enzymes of the gut microbiota can metabolize polyphenols producing smaller catabolite, which may be easily absorbed during intestinal transit. These catabolites may fall in two classes: some have higher biological activities compared with “parental” compound, and others lose biological activity [3].
Among polyphenols, curcumin has received great attention by researchers in the last years. Curcumin is normally found in the turmeric of Curcuma longa Linn. This plant belongs to the Zingiberaceae family and it is native from South Asia. Turmeric contains essential oils such as zingiberene and coloring agents that are known as curcuminoids [4]. Curcumin is the most important curcuminoid isolated from the rhizome of the plants. It has been used for centuries as a herbal remedy for the treatment of several diseases in East Asia due to its safety and intrinsic nontoxicity to humans, even at high doses [5] and nowadays it is widely used also in food, cosmetic, and pharmaceutical industries [6].
The general interest in its therapeutic efficacy arises from the different biological and pharmacological effects. In fact, curcumin possesses antioxidant and anti-inflammatory properties, and it has shown to exert beneficial effects against several types of cancers [7][8].
An increasing number of clinical trials based on curcumin administration have been published or are currently in progress, therefore demonstrating the expanding interest of the scientific community on the therapeutic potential of curcumin.
However, the pharmacological potential of curcumin is widely restricted because of its poor water solubility, chemical instability, and rapid metabolism. In addition, curcumin bioavailability is very low after oral administration [9]. In this regard, numerous attempts have been made to increase its efficacy and bioavailability.
2. Curcumin Metabolism
The bioavailability of curcumin, as with other polyphenols, is generally poor and after oral dosing, its blood levels are extremely low. The oral bioavailability of curcumin is poor due to a relatively low intestinal absorption and the rapid metabolism in the liver, followed by elimination through the gall bladder. Several studies reported very low levels of curcumin plasma concentration an hour after oral administration even after ingestion of high doses of curcumin [10].
Curcumin is used in different dosage depending on the disease. Recent in vitro studies report that curcumin is effective in reducing oxidative stress (OS) and in preventing neurodegeneration when used at a concentration ranging from 5–50 μM [11] and at a dose from 50 to 200 mg/Kg/day in vivo [12]. In clinical trials, curcumin resulted effective on oxidative stress and inflammation at a dosage from 90 to 2000 mg/day [13] while in neurodegenerative diseases prevention is evident after administration of 500–2000 mg [14].
Turmeric contains about 2%–9% of curcuminoids. Commercial turmeric extracts contain approximately 70%–75% curcumin, 20% demethoxycurcumin, and 5% bisdemethoxycurcumin. Curcumin shows a keto–enol tautomerism, and the balance between the two forms depends on the polarity and pH of the solvent with the keto and enol forms existing in given proportions. Once dissolved, the enol form predominates (Figure 1).
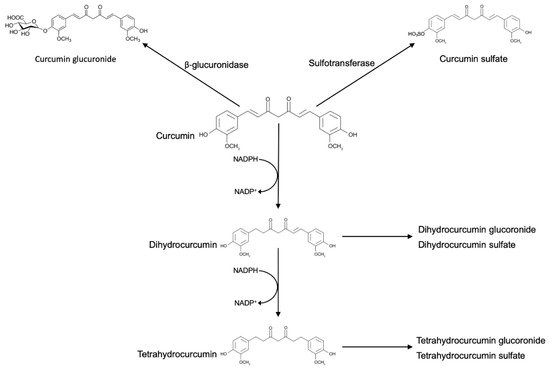
The bioavailability of curcumin has been studied extensively in mouse and humans. After oral administration of high doses, curcumin is poorly absorbed from the gastrointestinal tract, with peak blood levels rapidly reached within one hour after dosing [15].
Ingested curcumin first passes through the stomach, where the absorption of polyphenols is practically absent. Due to its resistance to low pH, curcumin reaches the large intestine without any chemical or structural modifications. In the large intestine, curcumin may be modified by phase I enzymes, a class of enzymes that introduces reactive and polar groups into their substrates. Phase I reactions that often produce active metabolites, yielded three metabolites, namely, tetrahydrocurcumin (M1), hexahydrocurcumin (M2), and octahydrocurcumin (M3). Then, curcumin and the phase I metabolites were subject to conjugation via phase II metabolism to yield their corresponding glucuronide and sulfate O-conjugated metabolites.
The result of these reactions is the increase of molecular weight and the production of less active metabolites than their substrates. In vitro and in vivo, curcumin and its reductive metabolites appear to be easily conjugated. Glucuronidation is the predominating pathway of conjugation, and the glucuronide of curcumin is usually found as the major metabolite of curcumin in body fluids, organs, and cells [16].
Commercial curcumin also contains demethoxycurcumin and bisdemethoxycurcumin, and these molecules undergo reductive metabolism very similar to curcumin, with the hexahydro product as the major metabolite and much smaller amounts of the octa-, tetra-, and dihydro products.
3. Biotransformation of Curcumin by Gut Microbiota
The transformation of curcumin is realized by enzymes of enterocytes and hepatocytes, but also by enzymes produced by the gut microbiota. Gut microbiota can be described as a biological reactor because of its own formidable metabolic functions, like the transformation of numerous compounds that reach the colon. This activity is made possible through the capacity of microorganisms for producing a huge and varied range of enzymes. In particular, curcumin intestinal transformations include several steps and different classes of microbial enzymes.Thus, the composition of the microbiota will cause different biotransformation of dietary curcumin.
Accordingly, the beneficial effects for consumers depend not only on the polyphenols taken from the diet, but also on the type of microbial population of the individual. Several enteric bacteria capable of modifying curcumin have been identified.
Curcumin can be modified in the colon tract by a specific microorganism, Escherichia coli. A specific enzyme converts curcumin first into the intermediate, dihhydrocurcumin, and then in the final product, tetrahydrocurcumin [17].
Another human intestinal bacteria strain, the Firmicute Blautia sp. (MRG-PMF1) is involved in curcumin metabolism. This bacterium produces two derivatives, demethylcurcumin and bisdemethylcurcumin by demethylation reaction [18]. Other microorganisms such as specific strains of Bifidobacteria (BB536, G4), Escherichia coli (K-12), Enterococcus faecalis (JCM 5803) and Lactobacillus acidophilus, and casei are all biologically relevant bacterial strains capable of degrading curcumin [19].
Recently, new curcumin metabolites produced by fecal bacteria have been identified. In particular, using specific assays a total of 23 metabolites were identified and also novel human gut microbiota curcumin metabolic pathways were discovered [20].
Interestingly, several evidences indicate that curcumin metabolites display a similar or superior potency to curcumin [35]. Indeed, tetrahydrocurcumin possesses superiority over curcumin as a free-radical quencher and it was shown to have therapeutic effects in neurodegenerative diseases through the inhibition of cytokines release or by inhibition of NF-κB activation [21].
4. Curcumin Effects on Gut Microbiota
In the intestine, curcumin, after oral or intraperitoneal administration, can exert a regulative effect on the gut microbiota community, affecting microbial richness, diversity, and composition. This effect should be involved in curcumin pharmacological activity [22] (Figure 2).
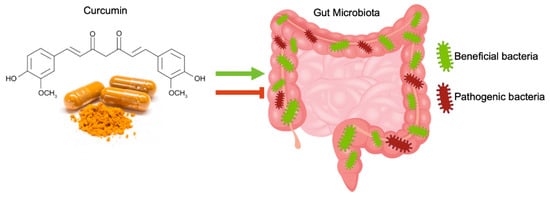
The administration of curcumin considerably changed the ratio between beneficial and pathogenic bacteria by increasing the abundance of Bifidobacteria, Lactobacillus, and reducing the loads of Prevotellaceae, Coriobacterales, Enterobacteria, and Enterococci [23].
Different studies report that oral administration of curcumin tends to decrease the microbial richness and diversity in mice and reduce the abundance of several representative bacterial families in gut microbial communities, such as Prevotellaceae, Bacteroidaceae, and Rikenellaceae, often associated to the onset of systemic diseases [24].
For example, a study evaluating the effects of curcumin on gut microbiota showed that in the placebo group, there was an overall reduction of bacterial species up to 15%. On the contrary, curcumin-treated subjects showed an increase of bacterial species up of 69%. Subjects responsive to the treatment had uniform increases in several bacterial species involved in the metabolism of curcumin. In addition, curcumin can exert prebiotic or probiotic effects depending on the individual efficiently of absorption. Thus, curcumin can change the composition of the microbiota, regulating not only the bacterial populations of the gut, but also the ability of intestinal bacterial strains to produce more active compounds from curcumin itself [25].
This entry is adapted from the peer-reviewed paper 10.3390/nu11102426
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10.
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Sahebkar, A.; Emami, S.A. Ethnobotany, phytochemistry and traditional uses of curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): A review. Curr. Pharm. Des. 2019, 25, 871–935.
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. Biofactors 2013, 39, 56–68.
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex®/Bioperine® has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Conundrum and Therapeutic Potential of Curcumin in Drug Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2010, 27, 279–312.
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499.
- Wang, X.; Gao, J.; Wang, Y.; Zhao, B.; Zhang, Y.; Han, F.; Zheng, Z.; Hu, D. Curcumin pretreatment prevents hydrogen peroxide-induced oxidative stress through enhanced mitochondrial function and deactivation of Akt/Erk signaling pathways in rat bone marrow mesenchymal stem cells. Mol. Cell. Biochem. 2018, 443, 37–45
- Wei, G.; Chen, B.; Lin, Q.; Li, Y.; Luo, L.; He, H.; Fu, H. Tetrahydrocurcumin Provides Neuroprotection in Experimental Traumatic Brain Injury and the Nrf2 Signaling Pathway as a Potential Mechanism. Neuroimmunomodulation 2017, 24, 348–355.
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 2017, 9, 187–208.
- Chen, M.; Du, Z.Y.; Zheng, X.; Li, D.L.; Zhou, R.P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752.
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702.
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494.
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620
- Burapan, S.; Kim, M.; Han, J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310.
- Jazayeri, S.D. Survival of Bifidobacteria and other selected intestinal bacteria in TPY medium supplemented with Curcumin as assessed in vitro. Int. J. Probiotics Prebiotics 2009, 4, 15–22.
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47.
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252.
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780.
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984.
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588.
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of turmeric and curcumin dietary supplementation on human gut microbiota: A double-blind, randomized, placebo-controlled pilot study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725.
