Bone tissue engineering (BTE) is a process of combining live osteoblast progenitors with a biocompatible scaffold to produce a biological substitute that can integrate into host bone tissue and recover its function. Mesenchymal stem cells (MSCs) are the most researched post-natal stem cells because they have self-renewal properties and a multi-differentiation capacity that can give rise to various cell lineages, including osteoblasts. BTE technology utilizes a combination of MSCs and biodegradable scaffold material, which provides a suitable environment for functional bone recovery and has been developed as a therapeutic approach to bone regeneration.
1. Introduction
Continuous research is ongoing in bone tissue regeneration technologies related to orthopedics and dentistry. Vast challenges remain, however, in the application of these modalities to reconstituting damaged skeletal structures. Bone grafting has been widely utilized as a regenerative therapy for critical size bone defects (CSDs), and various bone grafting and prosthetic bone materials have been developed in this regard. There is no one standard definition of CSDs. In general, a “critically-sized” defect is regarded as one that would not heal spontaneously within a patient’s lifetime and would require surgical stabilization and further surgical intervention [
1,
2]. Currently, bone grafting materials are classified as autogenous, allogeneic, or heterogeneous and artificial bone substitutes such as hydroxyapatite (HA), β-TCP (beta-tricalcium phosphate), bioactive glass, and calcium sulfate. Autologous bone has no particular disadvantages other than restrictions on the collection amount and collection site and is recognized as a good prosthetic material with new bone formation capacity. It is thus considered the current gold standard for the regeneration of bone defects but has been most widely used in clinics to treat only small-sized bone defects [
3,
4,
5].
To overcome the limitations of current bone graft therapies, such as autologous bone graft and artificial bone substitutes, many researchers have attempted to develop BTE to regenerate and restore lost bone tissue using MSCs, growth factors, and scaffolds [
6,
7,
8,
9,
10]. MSCs are referred to as multipotential progenitor cell populations that can differentiate into osteoblast progenitors in vitro under specific conditions, and these cells are most commonly used for bone regeneration [
11]. In addition, MSCs are immune tolerant and are used for immunosuppressive therapy via allogenic applications to accelerate bone healing [
12]. The use of a scaffold can provide the space needed to deliver and confine MSCs to the bone target site, provide an environment suitable for the migration, proliferation, and differentiation of the stem cells, enable diffusion of nutrients and eventually create early osteoid tissue at the site of the defect which is subsequently mineralized to form new bone. This combination of MSCs and scaffolds has been developed as a BTE therapy. Clinical trials for recovering bone defects have already commenced and reported the accelerated bone healing ability of these approaches. The current bone regenerating ability of the BTE approach is therefore successful but cannot as of yet recover the functional loss caused by large bone defects, such as those resulting from inflammatory diseases.
Osteoblasts are bone-forming cells derived from multipotent mesenchymal stem cells. During skeletal development, multipotent mesenchymal stem cells differentiate into osteoblast progenitor cells and undergo a commitment to form immature osteoblasts that are capable of proliferating before becoming mature osteoblasts. Although mature osteoblasts can synthesize and deposit bone extracellular matrix components, their ability to proliferate is significantly reduced [
13]. Thus, recapitulating immature osteoblast differentiation has been suggested as a potential approach to bone regeneration therapy [
14]. Currently, osteoblast progenitor cells can be isolated from adult human tissue and are good alternatives to MSCs for bone regeneration. BTE using immature osteoblast and bioscaffolds is, therefore an alternative tissue-engineered construct for recovering large bone defects.
2. MSCs Derived from Embryonic Stem Cells and Induced Pluripotent Stem Cells for Bone Tissue Regeneration
Functional bone tissue engineering generally involves the use of osteoprogenitors derived from MSCs and seeded onto a scaffold to predictably restore the lost architecture and function of bone tissue. MSCs have been isolated from adult tissues such as adipose tissue, bone marrow, and dental tissues, are widely used in regenerative medicine, including BTE, and, thus, have both research and clinical applications. However, MSCs cannot be isolated from patients with systemic disorders such as cardiovascular disease, diabetes, inflammatory bone disease, or advanced aging-related issues. Embryonic stem cell (ESCs)- or Induced pluripotent stem cell (iPSCs)-derived MSCs may be potential cell sources for the clinical trial of BTE [
17]. A better understanding of cell fate decisions and differentiation processes during osteoblast development may help to generate functional progenitor cells for tissue restoration. Over the years, technologies involving the osteoblast differentiation of ESCs and iPSCs have been significantly improved, and several studies have demonstrated the successful production of MSCs derived from ESCs/iPSCs for use in BTE therapies [
18,
19,
20] (
Figure 1).
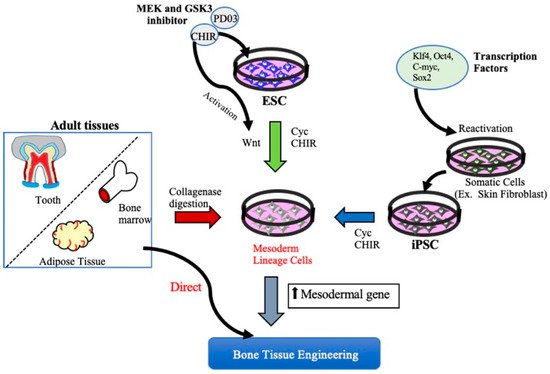
Figure 1. Schematic diagram of different approaches to obtain Mesenchymal stem cells. MSCs can be derived from either iPSCs, ESCs, or adult mesenchymal tissue. MSCs can be obtained by ESCs and iPSCs using small molecules such as mitogen-activated protein kinase (MEK) inhibitor, (MEK) inhibitor, PD0325901, glycogen synthase kinase 3 (GSK3) inhibitor, and CHIR99021 (CHIR). MSCs are also be derived from various connective tissues such as bone marrow, adipose tissue, and dental tissues by collagenase digestion or aspirates from bone marrow and adipose tissue directly used for BTE therapeutics. KLf4: Kruppel Like Factor 4, Oct4: Octamer-binding transcription factor 4, C-myc: Cellular-Myelocytomatosis, Sox-2: sex-determining region Y-box 2.
3. MSCs for Bone Regeneration
To develop MSCs that have clinical utility for BTE, a standard protocol for the characterization, osteoblast differentiation, and transplantation of these cells in combination with a biodegradable scaffold is required. Various types of MSCs are currently available with osteoblastic lineage differentiation potential; however, their origin and development are not clearly understood. There have been few reports on MSCs being successfully derived from neural crest cells during the development of vertebrates, which is seen as transient embryonic tissue [
29]. Most studies on MSCs to date have reported their derivation from perivascular cells, the pericytes. These cells reside in specific niches, which are commonly found in bone marrow, adipose tissue, and various fetal and other adult tissues [
30,
31]. This has been the primary cell source of the MSCs used in BTE to date.
3.1. Characterization of MSCs
Surface markers are currently being used to identify MSCs for quality control assurance prior to cell preparation, based on ‘good manufacturing practice,’ which is required for investor-mediated clinical developments. Hence, the characterization of MSCs based on surface marker analysis is an essential criterion for the clinical application of BTE methodologies. According to the International Society of Cell Therapy (ISCT) criteria, MSCs express a cluster of differentiation (CD) surface markers such as CD90, CD105, and CD73, but do not express CD11b, CD14, CD19, CD34, CD45, or human leukocyte antigen (HLA)-DR [
32,
33,
34].
3.2. Clinical Translation of MSC-Based Bone Regeneration
The basic concept behind a scaffold is to mimic the structure and function of the extracellular matrix (ECM) in tissues. The ECM provides both structural and mechanical stability and regulates some of the core cellular functions [42,43,44]. The basic role of scaffolds in BTE is to mimic the ECM of the native bone tissue and provide a functional three-dimensional space for the adhesion, migration, proliferation, and differentiation of osteoblast progenitors in which bone growth can occur [38,45,46,47]. An ideal scaffold for BTE should substitute for both the structure and function of the ECM and thus be capable of regenerating the lost bone tissue when seeded in conjunction with osteoblast progenitors. BTE innovations have led to the development of new biomaterials that resemble the 3D bone structure, in terms of mechanical properties as well as osteoconductive, osteoinductive, and osteogenic features [48,49]. Traditional bone repair approaches mainly focus on the use of bone grafts from autologous, allogeneic, and xenogeneic sources; however, complications such as donor-site morbidity and host immune rejection limit the application of these tissues [50]. The promise of BTE has principally involved overcoming these problems. The aims of BTE are to regenerate and restore the function of lost bone tissue using combinations of osteoblast progenitors and synthetic biomaterial scaffolds. Over the past decade, the use of synthetic biomaterials to enhance bone regeneration has significantly developed because of their capacity to mimic the natural environment of the extracellular matrix. The synthetic scaffold biomaterials predominantly used in BTE include calcium phosphate ceramics, biodegradable polymers, and composites, and the combination of ceramics and polymer scaffolds aims to utilize the properties of both materials [47,50,51].
3.2.2. Preclinical Studies of BTE in a Large Animal Model Using MSC/Scaffold Combinations
To translate the clinical use of MSCs combined with scaffolds for BTE, large animal model systems that closely resemble human physiology are required. A number of preclinical studies conducted using MSCs with varying combinations of biomaterials in critical bone defect models are listed in Table 1.
3.2.3. Gene Therapy for Bone Regeneration
Gene therapy is another promising approach for enhancing bone regeneration. Today, the advancement in life-sciences technology allows gene transfer technology to fabricate a tissue-engineered scaffold to accommodate the growth of genetically modified cells and the endogenous synthesis of desired gene products in a controlled manner. Gene therapy allows for the transfer of genetic material in the precise anatomic location of target cells, allowing the transgene expression from the cells with the currently available techniques [76]. Gene transfer can be performed by several ex vivo and in vivo delivery techniques and by either using viral (transduction) or non-viral (transfection) vectors [76,77]. Since this review is focused on the use of combined cell scaffold constructs for bone regeneration, we mainly discuss the ex vivo delivery method, which requires isolation of target cells and transfer of the desired gene to express the respective protein in vitro and then seeded onto the biocompatible carrier material to obtain cell-scaffold construct for bone tissue engineering applications. The two standard methods of ex vivo delivery include viral and non-viral, it being said that each type has its advantages and disadvantages. Viral vectors demonstrate high transfection efficiency with immunogenicity and toxicity, raising an issue of safety. In contrast, non-viral vectors usually consist of plasmid or related DNA, which are non-immunogenic and high safety but with low transfection efficiency [77,78]. Another promising approach is the sequential delivery of exogenous genes to promote the osteogenesis of stem cells. For example, genes that are expressed early and in the final stages of osteogenesis are different. Hence, delivering required osteogenic genes at specific time intervals into target cells induces efficient osteogenic differentiation. A recent study by Kim et al. demonstrated an effective sequential delivery of runt-related transcription factor 2 (RUNX2) and osterix genes induced conversion of human MSCs into pre-osteoblasts and subsequent delivery of activating transcription factor 4 (ATF4) gene triggered further osteogenesis. Differentiation of MSCs into desired mature cells can be regulated by the delivery time of specific osteogenic genes mimicking the natural process of bone remodeling [78,79].
3.2.4. Clinical Trials of MSCs for BTE
Over the past decade, a greater understanding has emerged with regard to the capabilities of MSCs to promote bone tissue regeneration, with numerous preclinical and clinical studies now underway. To identify the current potential combination of cell-scaffold constructs or tissue-engineered substitutes for bone tissue regeneration, we found twenty clinical trials. Nine are published (
Table 2), and others are listed in the ClinicalTrails.gov database (
Table 3). These trials have highlighted the importance of using cell-based therapy with various scaffolds to treat bone tissue regeneration in a real clinical setting. From the twenty identified clinical studies listed in
Table 2 and
Table 3, the majority report the use of BMMSCs, reflecting the fact that they are the most accepted cell source and the current gold standard in most clinical trials for treating bone disease, including nonunion fractures of long bones and craniofacial bone defects. However, in a few clinical trials, researchers have used umbilical cord (UC)- MSCs [
85], BMMSCs [
86], and adipose-derived MSCs as allogeneic cell sources to prepare the tissue-engineered constructs for regeneration of critical bone defects (NCT02307). Ceramic-based scaffolds are the primary choice in the majority of clinical trials, indicating their high clinical relevance. From the clinical trials listed in
Table 2 and
Table 3, most studies used a combination of BMMSCs with calcium-phosphate ceramics such as hydroxyapatite [
85,
87,
88], β-TCP [
86,
89] (NCT02803177, NCT02153372), biphasic calcium phosphate, a combination of hydroxyapatite and β-TCP [
90] (NCT04297813, NCT03325504, NCT01842477). Although most of these clinical trials used a simple combination of calcium-phosphate ceramics with BMMSCs, in a few studies, however, additional factors were included to facilitate enhanced bone regeneration. For example, Dilogo et al. added growth factor BMP2 along with cell scaffold constructs to enhance bone regeneration [
85,
88]. Similarly, researchers used BMMSCs mixed with BMP2 and loaded them on to 3-dimensional tissue-engineered collagen scaffold (NCT01958502) in another clinical trial. However, a clinical study by Baba et al. used polylactic scaffold seeded BMMSCs mixed with platelet-rich plasma solution and an additional 5000 units of human thrombin dissolved in 10% calcium chloride [
91].
4. Osteoblast-Based Bone Tissue Regeneration
In addition to the efforts to increase the bone-forming ability of MSCs as a cell source for bone tissue engineering, the use of osteoblasts that are capable of proliferating before maturing, and that can synthesize and deposit bone extracellular matrix components such as osteocalcin (OCN) and bone sialoprotein (BSP), provides a potential alternative BTE cell source for the treatment of large bone defects. However, since BTE is generally approached using a combination of osteoblasts induced from MSCs on biodegradable scaffolds, the resulting bone forming efficacy will be dependent on the differentiation potential of MSCs into osteoblasts. This could hamper the progress of BTE for treating large bone defects. There are two major mechanisms underlying skeletal development, intramembranous and endochondral ossification. In intramembranous ossification, osteoblast lineage cells, i.e., immature osteoblasts, are formed directly from condensed mesenchymal tissue. Endochondral ossification, by contrast, involves the production of osteochondral progenitors from MSCs that give rise to hyperchondrocytes which activate perichondrial cells to differentiate into immature osteoblasts. From the perspective of BTE, the formation of immature osteoblasts is the convergence point for both types of ossification.
5. Conclusions
Critical bone defects that cannot self-heal without a surgical intervention pose a significant challenge in the field of BTE. Compared to the traditional gold standard approach of using autogenous bone, regenerative methods will typically use either exogenous MSCs or immature osteoblasts seeded onto a bioactive scaffold placed at the defect area to regenerate functional bone. Adult MSCs from bone marrow and adipose tissue have often been used in various clinical studies for bone regeneration. The available data from both preclinical studies and clinical trials have shown promising results when BMMSCs are used as a cell source for bone tissue regeneration. Many clinical studies have also shown the beneficial effects of MSCs and scaffold combinations in bone healing. Although encouraging clinical results have been obtained by transplanting MSCs-scaffold constructs, the exact dosage and route of application remain to be optimized, and the fate of transplanted cells and their mechanisms of action need to be better monitored in more extensive future clinical trials. The development of an alternative immature osteoblast source combined with more effective scaffolds is also anticipated in the future. Immature osteoblasts have the ability to become a potential alternative cell source to adult MSCs, as they are osteogenic lineage-committed cells that enhance the efficacy of bone regeneration.
Immature osteoblasts can be obtained from bone tissue samples during routine oral surgical procedures from mandible/maxillary alveolar bone or surgeries involving long bones from the femur or tibia. The advantage of immature osteoblasts over MSCs is their spontaneous matrix formation upon transplantation without promoting osteogenic differentiation. Immature osteoblasts can directly secrete bone collagen matrix and release various factors such as M-CSF, RANKL, and VEGF, enhancing bone remodeling and bone regeneration in various BTE applications. The combination of an immature osteoblast culture system that possesses robust osteogenic activity and an appropriate biodegradable scaffold is an expected future BTE therapeutic option. This approach will facilitate the establishment of better clinical protocols for regeneration therapy in cases of large bone defects, as a treatment for orthopedic conditions such as back pain resulting from stenosis and lumbar spondylolisthesis osteosarcoma, and for horizontal alveolar bone defects in the dental field.
This entry is adapted from the peer-reviewed paper 10.3390/cells10102687