Even though the mechanisms by which exosomes are taken up by recipient cells is not fully understood, several studies bring evidence of a non-random process which is dependent on transmembrane proteins [
22,
23]. For example, a study by Kuroda et al. recently identified possible receptors for the uptake of exosomes derived from SK-Mel-28 melanoma cells in human brain capillary endothelial cells (hCMEC/D3) [
22]. This study revealed that the uptake of SK-Mel-28-derived exosomes by hCMEC/D3 cells occurs via macropinocytosis and receptor-mediated pathways, with major contribution of the presence of CD46 in hCMEC/D3 [
22]. This selective uptake by recipient cells together with the heavily regulated loading process briefly described in the previous section, supports the specific and critical function of these vesicles in intercellular communication.
2.2.1. The Tumor Microenvironment (TME): A Dynamic Neighborhood
The TME and its intrinsic complex intercellular communication network, established between stromal and cancer cells, highlights the magnitude of the challenge in understanding and treating cancer. The TME constantly changes during cancer progression as a response to evolving tumors and their oncogenic signals [
26]. Therefore, when addressing the formation of metastases, it is necessary to consider the influence of the TME, as its dynamic character allows tumor cells to modulate their own niche. This topic has been reviewed in detail by Quail and Joyce [
26].
Over the past decades, emerging evidence suggests that tumor-derived exosomes (TDEs) and exosomes derived from stromal cells of the TME are crucial in modulating tumor growth, angiogenesis, invasion, survival, and metastases formation [
27,
28]. Virtually, TDEs play critical roles in every step of the metastatic cascade. Overall, this process can be considered as having two different stages: the TME stage, where the TDEs induce the epithelial–mesenchymal transition (EMT) in neoplastic epithelial cells conferring them intravasation and migration ability; the PMN stage, which happens in distal and specific organs that will foster metastases [
29]. These two stages are represented in
Figure 1, which schematically represents the subject reviewed in this work. The role of exosomes in the PMN will be discussed in the next section. In fact, when trying to describe the role of exosomes in brain metastases formation, the most critical and intriguing step may be the transmigration of the BBB and further brain parenchyma colonization by cancer cells. However, before reaching that stage in the metastatic cascade, tumor cells need to first lose their adhesion to the surrounding stroma and enter the bloodstream [
29,
30].
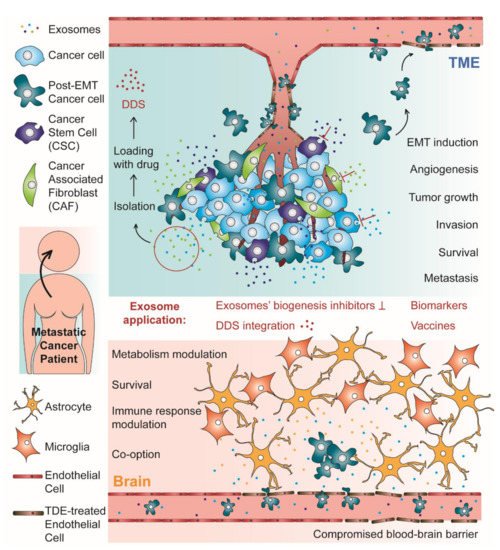
Figure 1. Schematic representation of both stages of the process of metastases formation with tropism to the brain: the tumor microenvironment (TME) and the pre-metastatic niche (PMN)/metastases establishment in the brain. In the TME, tumor derived exosomes (TDEs) are responsible for many critical phenomena, such as epithelial–mesenchymal transition (EMT) and angiogenesis, which supports tumor growth, invasion, survival, and metastases formation. In this stage, the exosomes can also induce endothelial barrier permeation, facilitating the passage of cancer cells to the blood flow. In the brain, exosomes from cancer cells induce many alterations that contribute to PMN establishment, such as blood–brain barrier (BBB) permeation, metabolism and immune response modulation and vascular co-option induction, which supports the brain parenchyma invasion by arriving circulating tumor cells, metastases formation and cancer cells survival. Brain metastases are also supported by the crosstalk between cancer cells and cells from the BBB, such as astrocytes. For their critical role in metastases formation, exosomes can be considered as a promising new target for metastatic cancer therapy, using inhibitors of exosomes’ biogenesis. Alternatively, these extracellular vesicles have been investigated as biomarkers for metastatic cancer diagnosis and prognosis, as vehicles in drug delivery systems (DDS) and as cell-free therapeutic tools in anti-tumor vaccination.
The metastatic cascade is initiated within the TME, with the activation of EMT process in neoplastic epithelial cells [
29]. EMT is the reversible process by which a neoplastic epithelial cell undergoes to acquire mesenchymal features, such as migratory and invasive abilities [
29]. During this process, the cells undergoing EMT downregulate epithelial markers, such as cytokeratin and E-cadherin, and upregulate mesenchymal markers as N-cadherin and vimentin [
31,
32]. Interestingly, the cadherin switch inherent to EMT modulates pro- and anti-apoptotic genes, allowing cancer cells to avoid programmed cell death induced by adhesion loss once they acquire a mesenchymal phenotype [
32]. Other relevant features of the mesenchymal phenotype is the increase of matrix metalloproteinases (MMPs) and altered protein production which contributes to the breakdown of the basement membrane [
33,
34]. Overall, the mesenchymal phenotype renders neoplastic cells the ability to alter their shape and motility, detach from the primary tumor site and enter the bloodstream [
35]. Additionally, exosomes secreted during hypoxia, which is linked to EMT and high risk of metastases, are enriched in EMT inducers when compared to those produced in a normoxic state [
29].
Recent studies have been building evidence of TDEs involvement in EMT. More specifically, TDEs have been described to transfer considerable amounts of EMT inducers to recipient tumor stroma epithelial cells, which then undergo biochemical changes consistent with EMT [
29,
36,
37,
38]. The EMT induction promoted by exosomes can happen via several EMT-related signaling pathways. One of the most studied EMT-inducing signaling pathways is Wnt/β-catenin which is also a common target for exosomes [
39,
40]. For instance, the transfer of exosomal microRNA (miR)-1260b between lung adenocarcinoma cells leads to downregulation of sFRP1 and Smad4, activating the Wnt/β-catenin pathway [
41]. Exosomes derived from cancer associated fibroblasts (CAFs) transferred miR-92a-3p to colorectal cancer cells activating the Wnt/β-catenin pathway and thereby inducing EMT [
42]. Furthermore, exosomal miR-92a-3p effect in recipient cells also included apoptosis inhibition and chemotherapy resistance [
42], which demonstrates that the crosstalk within the TME dictates tumor progression by modulating several features within the tumor niche. Another line of evidence supporting this TME modulation through exosome-mediated crosstalk is the transfer of miR-155 from breast cancer stem cells (CSCs) and chemoresistant breast cancer cells to sensitive breast cancer cells, leading to marked chemoresistance and inducing EMT [
36]. Additionally, a study by Donnaruma and co-workers revealed that several exosomal miRNAs secreted by CAFs were able to induce EMT, facilitate anchorage-independent cell growth and increase the ability to form mammospheres in breast cancer cells [
43]. In addition to miRNAs, You and co-workers recently showed that CAFs derived exosomes transferred SNAI1 mRNA to lung cancer cells, inducing EMT via Wnt/β-catenin pathway [
44]. Snail1 is a transcription factor which represses the expression of E-cadherin, therefore inducing EMT [
44]. In another study, Menck and co-workers described the reciprocal loop between infiltrating macrophages and breast cancer cells. Breast cancer cells secrete exosomes that induce the Wnt ligand Wnt5a in infiltrating macrophages. Macrophages are then responsible to shuttle the Wnt5a to tumor cells, promoting their invasion [
45].
Alternatively, exosomes secreted by mesenchymal stem cells (MSCs)-derived adipocytes were able to induce EMT in breast cancer cells via Hippo pathway [
46]. Even though in this case the cargo responsible for EMT induction was not identified, other studies showed that activation of the Hippo pathway may result from the transfer of exosomal miRNAs or proteins [
47,
48,
49,
50]. Another alternative pathway associated with EMT is the extracellular-regulated protein kinase (ERK) pathway. Exosomes secreted by gastric cancer cells activated the mitogen-activated protein kinase (MAPK)/ERK pathway in recipient cells, leading to tumor proliferation [
51].
Collectively, these studies are evidence that the exosome-mediated crosstalk within the TME is crucial for the initiation of metastization process, involving not only TDEs but also exosomes derived from CAFs, macrophages and many other cell types.