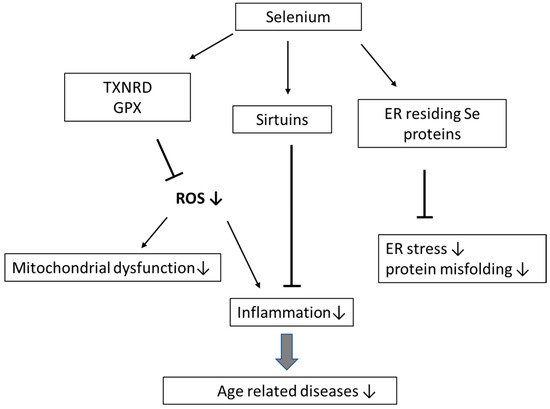
Selenium (Se) is an essential dietary trace element that plays an important role in the prevention of inflammation, cardiovascular diseases, infections, and cancer. Selenoproteins contain selenocysteine in the active center and include, i.a., the enzymes thioredoxin reductases (TXNRD1–3), glutathione peroxidases (GPX1–4 and GPX6) and methionine sulfoxide reductase, involved in immune functions, metabolic homeostasis, and antioxidant defense. Ageing is an inevitable process, which, i.a., involves an imbalance between antioxidative defense and reactive oxygen species (ROS), changes in protein and mitochondrial renewal, telomere attrition, cellular senescence, epigenetic alterations, and stem cell exhaustion. These conditions are associated with mild to moderate inflammation, which always accompanies the process of ageing and age-related diseases. In older individuals, Se, by being a component in protective enzymes, operates by decreasing ROS-mediated inflammation, removing misfolded proteins, decreasing DNA damage, and promoting telomere length. Se-dependent GPX1–4 and TXNRD1–3 directly suppress oxidative stress. Selenoprotein H in the cell nucleus protects DNA, and selenoproteins residing in the endoplasmic reticulum (ER) assist in the removal of misfolded proteins and protection against ER stress.
- selenium
- ageing
- cardiovascular
- cancer
- sirtuins
- telomeres
1. Introduction
2. Selenium Nutrition: From Basic to Clinical Aspects
3. Selenium Deficiency—A Role in Diseases in the Elderly
3.1. Ageing and Inflammation
3.2. Selenium, Ageing and Cardiovascular Disease (CVD)
3.3. Selenium, Ageing and Neurodegenerative Diseases
3.4. Selenium, Ageing and Cancer
3.5. Selenium, Ageing, and Other Age-Related Diseases
This entry is adapted from the peer-reviewed paper 10.3390/biom11101478
References
- Afanas’ev, I.B. Free radical mechanisms of aging processes under physiological conditions. Biogerontology 2005, 6, 283–290.
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33.
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74.
- Zhang, Z.; Liu, J.; Rozovsky, S. Preparation of Selenocysteine-Containing Forms of Human SELENOK and SELENOS. Methods Mol. Biol. 2018, 1661, 241–263.
- Patel, C.; Saad, H.; Shenkman, M.; Lederkremer, G.Z. Oxidoreductases in Glycoprotein Glycosylation, Folding, and ERAD. Cells 2020, 9, 2138.
- Mocchegiani, E.; Malavolta, M.; Muti, E.; Costarelli, L.; Cipriano, C.; Piacenza, F.; Tesei, S.; Giacconi, R.; Lattanzio, F. Zinc, metallothioneins and longevity: Interrelationships with niacin and selenium. Curr. Pharm. Des. 2008, 14, 2719–2732.
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal 2013, 19, 546–558.
- Alehagen, U.; Aaseth, J.; Lindahl, T.L.; Larsson, A.; Alexander, J. Dietary Supplementation with Selenium and Coenzyme Q10 Prevents Increase in Plasma D-Dimer While Lowering Cardiovascular Mortality in an Elderly Swedish Population. Nutrients 2021, 13, 1344.
- Akbaraly, N.T.; Arnaud, J.; Hininger-Favier, I.; Gourlet, V.; Roussel, A.M.; Berr, C. Selenium and mortality in the elderly: Results from the EVA study. Clin. Chem. 2005, 51, 2117–2123.
- Gonzalez, S.; Huerta, J.M.; Fernandez, S.; Patterson, A.M.; Lasheras, C. Life-quality indicators in elderly people are influenced by selenium status. Aging Clin. Exp. Res. 2007, 19, 10–15.
- Johansson, P.; Dahlstrom, O.; Dahlstrom, U.; Alehagen, U. Improved Health-Related Quality of Life, and More Days out of Hospital with Supplementation with Selenium and Coenzyme Q10 Combined. Results from a Double Blind, Placebo-Controlled Prospective Study. J. Nutr. Health Aging 2015, 19, 870–877.
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Dahlstrom, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866.
- Ray, A.L.; Semba, R.D.; Walston, J.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Xue, Q.L.; Fried, L.P. Low serum selenium and total carotenoids predict mortality among older women living in the community: The women’s health and aging studies. J. Nutr. 2006, 136, 172–176.
- Foster, H.D.; Zhang, L. Longevity and selenium deficiency: Evidence from the People’s Republic of China. Sci. Total Environ. 1995, 170, 133–139.
- Huang, Y.; Rosenberg, M.; Hou, L.; Hu, M. Relationships among Environment, Climate, and Longevity in China. Int. J. Environ. Res. Public Health 2017, 14, 1195.
- Santesmasses, D.; Castro, J.P.; Zenin, A.A.; Shindyapina, A.V.; Gerashchenko, M.V.; Zhang, B.; Kerepesi, C.; Yim, S.H.; Fedichev, P.O.; Gladyshev, V.N. COVID-19 is an emergent disease of aging. Aging Cell 2020, 19, e13230.
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358.
- Bjorklund, G.; Aaseth, J.; Ajsuvakova, O.P.; Nikonorov, A.A.; Skalny, A.; Skalnaya, M.G.; Tinkov, A. Molecular interaction between mercury and selenium in neurotoxicity. Coord. Chem. Rev. 2017, 332, 30–37.
- Reeves, M.A.; Hoffmann, P.R. The human selenoproteome: Recent insights into functions and regulation. Cell. Mol. Life Sci. 2009, 66, 2457–2478.
- Dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratao, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. In Selenium in Plants: Plant Ecophysiology; Pilon-Smits, E., Winkel, L., Lin, Z.Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 11, pp. 209–230.
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134.
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid Redox Signal 2011, 14, 1337–1383.
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531.
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047.
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4.
- Flohe, L. The glutathione peroxidase reaction: Molecular basis of the antioxidant function of selenium in mammals. Curr. Top. Cell. Regul. 1985, 27, 473–478.
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal 2018, 29, 61–74.
- Tinkov, A.A.; Bjorklund, G.; Skalny, A.V.; Holmgren, A.; Skalnaya, M.G.; Chirumbolo, S.; Aaseth, J. The role of the thioredoxin/thioredoxin reductase system in the metabolic syndrome: Towards a possible prognostic marker? Cell. Mol. Life Sci. 2018, 75, 1567–1586.
- Alehagen, U.; Aaseth, J.; Johansson, P. Reduced Cardiovascular Mortality 10 Years after Supplementation with Selenium and Coenzyme Q10 for Four Years: Follow-Up Results of a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly Citizens. PLoS ONE 2015, 10, e0141641.
- Aaseth, J.; Alexander, J.; Alehagen, U. Coenzyme Q10 supplementation—In ageing and disease. Mech. Ageing Dev. 2021, 197, 111521.
- Bell, H.S.; Tower, J. In vivo assay and modelling of protein and mitochondrial turnover during aging. Fly 2021, 15, 60–72.
- Emwas, A.H.; Alghrably, M.; Dhahri, M.; Sharfalddin, A.; Alsiary, R.; Jaremko, M.; Faa, G.; Campagna, M.; Congiu, T.; Piras, M.; et al. Living with the enemy: From protein-misfolding pathologies we know, to those we want to know. Ageing Res. Rev. 2021, 70, 101391.
- Rocca, C.; Pasqua, T.; Boukhzar, L.; Anouar, Y.; Angelone, T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum-resident selenoproteins. Cell. Mol. Life Sci. 2019, 76, 3969–3985.
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium Deficiency Is Associated with Pro-longevity Mechanisms. Cell Rep 2019, 27, 2785–2797.e3.
- Wu, R.T.; Cao, L.; Mattson, E.; Witwer, K.W.; Cao, J.; Zeng, H.; He, X.; Combs, G.F., Jr.; Cheng, W.H. Opposing impacts on healthspan and longevity by limiting dietary selenium in telomere dysfunctional mice. Aging Cell 2017, 16, 125–135.
- Ran, Q.; Liang, H.; Ikeno, Y.; Qi, W.; Prolla, T.A.; Roberts, L.J. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 932–942.
- Xu, J.W.; Shi, X.M.; Yin, Z.X.; Liu, Y.Z.; Zhai, Y.; Zeng, Y. Investigation and analysis of plasma trace elements of oldest elderly in longevity areas in China. Zhonghua Yu Fang Yi Xue Za Zhi 2010, 44, 119–122.
- Hao, Z.; Liu, Y.; Li, Y.; Song, W.; Yu, J.; Li, H.; Wang, W. Association between Longevity and Element Levels in Food and Drinking Water of Typical Chinese Longevity Area. J. Nutr. Health Aging 2016, 20, 897–903.
- Cesari, M.; Penninx, B.W.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Rhys Williams, G.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 242–248.
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105.
- Alehagen, U.; Aaseth, J.; Johansson, P. Less increase of copeptin and MR-proADM due to intervention with selenium and coenzyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors 2015, 41, 443–452.
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390.
- Ragy, M.M.; Kamal, N.N. Linking senile dementia to type 2 diabetes: Role of oxidative stress markers, C-reactive protein and tumor necrosis factor-alpha. Neurol. Res. 2017, 39, 587–595.
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695.
- Alehagen, U.; Lindahl, T.L.; Aaseth, J.; Svensson, E.; Johansson, P. Levels of sP-selectin and hs-CRP Decrease with Dietary Intervention with Selenium and Coenzyme Q10 Combined: A Secondary Analysis of a Randomized Clinical Trial. PLoS ONE 2015, 10, e0137680.
- Alehagen, U.; Alexander, J.; Aaseth, J.; Larsson, A. Decrease in inflammatory biomarker concentration by intervention with selenium and coenzyme Q10: A subanalysis of osteopontin, osteoprotergerin, TNFr1, TNFr2 and TWEAK. J. Inflamm. 2019, 16, 5.
- Rancano, K.M.; Ralston, P.A.; Lemacks, J.L.; Young-Clark, I.; Ilich, J.Z. Antioxidant intake in relation to serum C-reactive protein in mid-life and older African Americans. Ethn. Health 2020, 25, 1132–1144.
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Dall’Aglio, E.; Guralnik, J.M.; Paolisso, G.; Semba, R.D.; Nouvenne, A.; Borghi, L.; et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: The InCHIANTI study. Clin. Nutr. 2010, 29, 674–677.
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Brismar, K. Increase in insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 1 after supplementation with selenium and coenzyme Q10. A prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS ONE 2017, 12, e0178614.
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476.
- Shahgaldi, S.; Kahmini, F.R. A comprehensive review of Sirtuins: With a major focus on redox homeostasis and metabolism. Life Sci. 2021, 282, 119803.
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238.
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009, 136, 62–74.
- Giacconi, R.; Chiodi, L.; Boccoli, G.; Costarelli, L.; Piacenza, F.; Provinciali, M.; Malavolta, M. Reduced levels of plasma selenium are associated with increased inflammation and cardiovascular disease in an Italian elderly population. Exp. Gerontol. 2021, 145, 111219.
- Salonen, J.T.; Alfthan, G.; Huttunen, J.K.; Pikkarainen, J.; Puska, P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet 1982, 2, 175–179.
- Suadicani, P.; Hein, H.O.; Gyntelberg, F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis 1992, 96, 33–42.
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402.
- Huttunen, J.K. Selenium and cardiovascular diseases—An update. Biomed. Environ. Sci. 1997, 10, 220–226.
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241.
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613.
- Alehagen, U.; Aaseth, J. Selenium and coenzyme Q10 interrelationship in cardiovascular diseases—A clinician’s point of view. J. Trace Elem. Med. Biol. 2015, 31, 157–162.
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE 2016, 11, e0157541.
- Kardinaal, A.F.; Kok, F.J.; Kohlmeier, L.; Martin-Moreno, J.M.; Ringstad, J.; Gomez-Aracena, J.; Mazaev, V.P.; Thamm, M.; Martin, B.C.; Aro, A.; et al. Association between toenail selenium and risk of acute myocardial infarction in European men. The EURAMIC Study. European Antioxidant Myocardial Infarction and Breast Cancer. Am. J. Epidemiol. 1997, 145, 373–379.
- Bomer, N.; Grote Beverborg, N.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; Ijmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2020, 22, 1415–1423.
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients 2019, 11, 1852.
- Salvini, S.; Hennekens, C.H.; Morris, J.S.; Willett, W.C.; Stampfer, M.J. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians. Am. J. Cardiol. 1995, 76, 1218–1221.
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773.
- Kuria, A.; Tian, H.; Li, M.; Wang, Y.; Aaseth, J.O.; Zang, J.; Cao, Y. Selenium status in the body and cardiovascular disease: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–10.
- Rees, K.; Hartley, L.; Day, C.; Clarke, A.; Stranges, S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2012, 2012.
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.M.; Favier, A.; Briancon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342.
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96.
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795.
- Aaseth, J.; Alexander, J.; Bjorklund, G.; Hestad, K.; Dusek, P.; Roos, P.M.; Alehagen, U. Treatment strategies in Alzheimer’s disease: A review with focus on selenium supplementation. Biometals 2016, 29, 827–839.
- Varikasuvu, S.R.; Prasad, V.S.; Kothapalli, J.; Manne, M. Brain Selenium in Alzheimer’s Disease (BRAIN SEAD Study): A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2019, 189, 361–369.
- Solovyev, N. Selenoprotein P and its potential role in Alzheimer’s disease. Hormones 2020, 19, 73–79.
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447.
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573.
- Du, X.; Wang, C.; Liu, Q. Potential Roles of Selenium and Selenoproteins in the Prevention of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 835–848.
- Shahar, A.; Patel, K.V.; Semba, R.D.; Bandinelli, S.; Shahar, D.R.; Ferrucci, L.; Guralnik, J.M. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov. Disord. 2010, 25, 1909–1915.
- Akbaraly, T.N.; Hininger-Favier, I.; Carriere, I.; Arnaud, J.; Gourlet, V.; Roussel, A.M.; Berr, C. Plasma selenium over time and cognitive decline in the elderly. Epidemiology 2007, 18, 52–58.
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228.
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535.
- Ellwanger, J.H.; Franke, S.I.; Bordin, D.L.; Pra, D.; Henriques, J.A. Biological functions of selenium and its potential influence on Parkinson’s disease. An. Acad. Bras. Cienc. 2016, 88, 1655–1674.
- Zafar, K.S.; Siddiqui, A.; Sayeed, I.; Ahmad, M.; Salim, S.; Islam, F. Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: Neurobehavioral and neurochemical evidences. J. Neurochem. 2003, 84, 438–446.
- Sun, H. Association of soil potassium and sodium concentrations with spatial disparities of prevalence and mortality rates of hypertensive diseases in the USA. Environ. Geochem. Health 2018, 40, 1513–1524.
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit. Rev. Food Sci. Nutr. 2020, 60, 684–694.
- Aro, A.; Alfthan, G.; Varo, P. Effects of supplementation of fertilizers on human selenium status in Finland. Analyst 1995, 120, 841–843.
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963.
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51.
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106, djt456.
- Combs, G.F., Jr.; Clark, L.C.; Turnbull, B.W. An analysis of cancer prevention by selenium. Biofactors 2001, 14, 153–159.
- Nomura, A.M.; Lee, J.; Stemmermann, G.N.; Combs, G.F., Jr. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 883–887.
- Allen, N.E.; Appleby, P.N.; Roddam, A.W.; Tjonneland, A.; Johnsen, N.F.; Overvad, K.; Boeing, H.; Weikert, S.; Kaaks, R.; Linseisen, J.; et al. Plasma selenium concentration and prostate cancer risk: Results from the European prospective investigation into cancer and nutrition (EPIC). Am. J. Clin. Nutr. 2008, 88, 1567–1575.
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Meplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2015, 136, 1149–1161.
- Cabral, M.; Kuxhaus, O.; Eichelmann, F.; Kopp, J.F.; Alker, W.; Hackler, J.; Kipp, A.P.; Schwerdtle, T.; Haase, H.; Schomburg, L.; et al. Trace element profile and incidence of type 2 diabetes, cardiovascular disease and colorectal cancer: Results from the EPIC-Potsdam cohort study. Eur. J. Nutr. 2021, 60, 3267–3278.
- Hughes, D.J.; Duarte-Salles, T.; Hybsier, S.; Trichopoulou, A.; Stepien, M.; Aleksandrova, K.; Overvad, K.; Tjonneland, A.; Olsen, A.; Affret, A.; et al. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2016, 104, 406–414.
- Salonen, J.T.; Salonen, R.; Lappetelainen, R.; Maenpaa, P.H.; Alfthan, G.; Puska, P. Risk of cancer in relation to serum concentrations of selenium and vitamins A and E: Matched case-control analysis of prospective data. Br. Med. J. 1985, 290, 417–420.
- Peters, U.; Takata, Y. Selenium and the prevention of prostate and colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1261–1272.
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253.
- Ho, E.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv. Nutr. 2011, 2, 497–510.
- Libby, P.; Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc. Res. 2019, 115, 824–829.
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes with the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28.
- Alehagen, U.; Aaseth, J.; Alexander, J.; Brismar, K.; Larsson, A. Selenium and Coenzyme Q10 Supplementation Improves Renal Function in Elderly Deficient in Selenium: Observational Results and Results from a Subgroup Analysis of a Prospective Randomised Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 3780.
- Pakfetrat, M.; Malekmakan, L.; Hasheminasab, M. Diminished selenium levels in hemodialysis and continuous ambulatory peritoneal dialysis patients. Biol. Trace Elem. Res. 2010, 137, 335–339.
- Reinhardt, W.; Dolff, S.; Benson, S.; Broecker-Preuss, M.; Behrendt, S.; Hog, A.; Fuhrer, D.; Schomburg, L.; Kohrle, J. Chronic Kidney Disease Distinctly Affects Relationship Between Selenoprotein P Status and Serum Thyroid Hormone Parameters. Thyroid 2015, 25, 1091–1096.
- Stockler-Pinto, M.B.; Malm, O.; Moraes, C.; Farage, N.E.; Silva, W.S.; Cozzolino, S.M.; Mafra, D. A follow-up study of the chronic kidney disease patients treated with Brazil nut: Focus on inflammation and oxidative stress. Biol. Trace Elem. Res. 2015, 163, 67–72.
