1. Ghrelin and Its Synthesis
Ghrelin, a 28-amino acid peptide, was primary isolated by Kojima et al. from rat and human stomachs in 1999 [
1,
2,
3]. The main source of endogenous ghrelin in the body is the stomach [
1,
4]. Ghrelin is created from its 117-amino acid precursor, preproghrelin, which consists of a 23-amino acid signal sequence and the 94-amino acid proghrelin [
1,
5]. The proghrelin is further converted into acyl-ghrelin, des-acyl ghrelin, and obestatin [
5,
6,
7].
Most studies show that the majority of ghrelin in circulation exists in the form of des-acyl ghrelin [
8,
9,
10]. On the other hand, Blatnik et al. [
11] postulate that these observations are a result of errors in sampling, handling, collection, and assessment of serum ghrelin. Blatnik et al. analyzed the acyl ghrelin plasma stability by LC-MS/MS and revealed that acyl ghrelin is enzymatically and chemically converted to des-acyl ghrelin in the presence of active serine proteases and HCl. They concluded that that normally all circulating ghrelin is acylated, and des-acyl ghrelin should not be detectible in healthy human plasma under optimal sample handling and assaying conditions [
11].
Acyl-ghrelin is considered to be an active form of this hormone [
6,
8,
12]. Acylation is necessary to stimulate the growth hormone secretagogue receptor (GHSR-1a), currently known as the ghrelin receptor [
13]. The ghrelin receptors are mainly expressed in the pituitary gland and hypothalamus, but were also present in other tissues and organs [
5,
13,
14,
15]. Expression of ghrelin receptor is highly sensitive to the level of growth hormone. In growth hormone-deficient animals, expression of mRNA for ghrelin receptor is increased. On the other hand, an increase in serum growth hormone level reduces the expression of ghrelin receptor [
16].
Acylation of ghrelin is catalyzed by the ghrelin O-acyltransferase (GOAT), which was discovered in 2008 [
17]. GOAT belongs to a family of hydrophobic membrane-bound acyltransferases [
17,
18]. Des-acyl ghrelin does not bind to ghrelin receptor, GHSR-1a, and is deprived of growth hormone releasing activity. However, this form of ghrelin may exhibit some non-endocrinological activity, such as the protection of endothelial cells and cardiomyocytes in the heart, regulation of food intake, gastric and pancreatic secretion, gut motility, adipogenesis, stimulation of bone formation, insulin secretion, and prevention of skeletal muscle atrophy [
2,
3,
19,
20].
Acyl-ghrelin acting on ghrelin receptor (previously known as GHSR-1a) strongly and dose-dependently stimulates synthesis and release of growth hormone in the anterior lobe of the pituitary gland [
1,
3]. This effect of ghrelin is mainly related to direct stimulation of somatotropes. However, ghrelin also stimulates the liberation of growth hormone via an indirect pathway. Ghrelin, acting on neurons expressing growth hormone-releasing hormone (GH-RH) in the hypothalamus, leads to the secretion of GH-RH by these neurons. Subsequently, GH-RH reaches somatotropes in the anterior part of the pituitary and stimulates them to release the growth hormone [
21]. The ghrelin receptor is a G-protein-coupled receptor and signals via a Gq/11 alpha-subunit, that results in the activation of phospholipase C and the synthesis of inositol triphosphate (IP3), and releases Ca2+ from the endoplasmic reticulum [
12,
22]. On the other hand, Ge et al. [
23] have reported that stimulatory effect of ghrelin on ghrelin receptor can be reduced by liver-expressed antimicrobial peptide 2 (LEAP2), an endogenous antagonist of ghrelin receptor. LEAP2 is produced in the liver and small intestine. This peptide inhibits ghrelin receptor activation by ghrelin, leading to reduction in the major effects of ghrelin in the body, such as food intake, growth hormone release, and maintenance of viable glucose levels during fasting. Secretion of endogenous LEAP2 is suppressed by food restriction, and this effect leads to increased reactivity of ghrelin receptor to the action of ghrelin [
23]. Moreover, studies performed on neoplastic cell lines suggest that ghrelin may activate P13K/GTP-Rac [
24], GHSR/P13K/Akt [
25], and GHSR/CaMKII/AMPK/NFκB [
26] signaling pathways.
Apart from ghrelin receptor, there is another type of growth hormone secretagogue receptor, GHSR-1b, but this receptor seems to be not biologically active. Its role is unknown [
3].
Ghrelin is mainly synthesized in the gastric oxyntic mucosa, but its presence was also found in the oral cavity, small and large bowel, pancreas, thyroid, lung, testis, myocardium, kidney, brain cortex, brain stem, pituitary, hypothalamus, and immune cells [
14,
15,
27,
28]. In rats and dogs, ghrelin is produced in the stomach by the neuroendocrine X/A-like cells [
29,
30]. These cells are small and round. They have no contact with a stomach lumen. In the human stomach, ghrelin is produced in endocrine cells called P/D1 cells. In the small and large bowel, there are two types of ghrelin-secreting cells: closed-type cells with triangular and elongated shapes, and opened-type cells with their apical cytoplasmic process contacting to the intestinal lumen [
1,
30]. In the pancreas, ghrelin is produced by endocrine and exocrine cells [
15,
31,
32].
In the case of a decrease in the production of ghrelin in the gastric mucosa, a compensatory increase in the production of this peptide in other areas of the body may occur. Partial resection of gastric mucosa, as a result of bariatric surgery leads to a decrease in serum ghrelin level in the early postoperative period [
33]. Later, however, this level returns to the initial value [
33] or may be even higher than before the operation [
34]. In line with those observation are findings of animal studies performed by Camacho-Ramirez et al. [
35], who found that a severe reduction in gastric secretion of ghrelin leads to an increase in the islet ghrelin-secreting epsilon cell population, leading to a subsequent recovery of basal serum ghrelin levels.
2. Physiological Action of Ghrelin
The main physiological function of ghrelin is dose-dependent stimulation of growth hormone release from the pituitary gland [
1,
2]. The endocrine effects of ghrelin also include the stimulation of prolactin, cortisol, and adrenocorticotropic hormone secretion [
36,
37].
Ghrelin is responsible for a positive energy balance. This hormone increases food intake and fat deposition [
2,
38,
39]. The increase in appetite, known as orexigenic effect, is mediated by stimulation of hypothalamic neurons releasing neuropeptide Y, orexin, and agouti-related protein (AgRP), as well as by inhibition of hypothalamic proopiomelanocortin (POMC) neurons [
40,
41,
42]. Among orexigenic peptides stimulating appetite, ghrelin is the only one acting peripherally, whereas all other orexigenic peptides are acting centrally [
12]. Besides the stimulation of food intake, ghrelin promotes carbohydrate oxidation and inhibits fat utilization, leading to positive energy balance [
43]. The plasma level of ghrelin is negatively correlated with BMI and food intake. For this reason, the plasma concentration of ghrelin is enhanced by anorexia nervosa, starvation, and cachexia, while obesity leads to the opposite effect [
44]. Food intake decreases the plasma ghrelin levels, however the degree of this reduction depends on the type of nutrients present in the food. The strongest effect is observed after protein consumption, smaller in case of carbohydrates, and the smallest after the ingestion of lipids [
45] (
Figure 1).
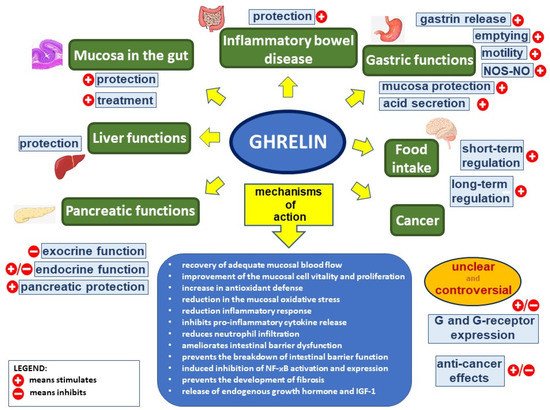
Figure 1. Ghrelin’s effect in the digestive system. Figure legend: NOS–NO—nitric oxide synthase–nitric oxide, G—ghrelin, NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells, and IGF-1—insulin-like growth factor-1; (+) means stimulates, (−) means inhibits.
Ghrelin stimulates gastric motility and gastric emptying [
2,
46,
47]. Impact of ghrelin on the exocrine secretory activity in the stomach is unclear. Gastric acid secretion is dose-dependently increased by the ghrelin administrated peripherally, through a mechanism involving vagal nerve activity and histamine release [
46,
48,
49,
50]. Ghrelin effects on gastric acid secretion are in synergy with effects of gastrin [
12,
51,
52]. On the other hand, ghrelin administrated centrally exhibits the opposite effect, inhibiting gastric acid release [
12,
53,
54].
Circulating ghrelin inhibits pancreatic exocrine secretion. Zhang et al. [
55] demonstrated that intravenous administration of ghrelin reduces the 2-deoxy-D-glucose- and cholecystokinin-stimulated pancreatic exocrine secretion in anesthetized rats. Moreover, ghrelin inhibits the potassium-stimulated amylase secretion in isolated pancreatic lobules [
2,
55]. On the other hand, Sato et al. [
56] reported, that intracerebroventricular administration of ghrelin rises pancreatic exocrine secretion in conscious rats, and the mechanism of this effect involves the vagal nerves [
2,
56]. The effect of ghrelin on pancreatic endocrine secretion was initially unclear. Early studies have shown that ghrelin increases insulin secretion by pancreatic β-cells [
44,
57,
58], while next studies have reported that ghrelin inhibits insulin release in the islets of Langerhans [
44,
59,
60]. Currently, it is commonly accepted that ghrelin inhibits glucose-dependent insulin secretion, acting directly on beta-cells in pancreatic islets [
44,
61,
62]. Physiologically, this mechanism is mainly related to ghrelin expressed in pancreatic islets and released into pancreatic microcirculations. Ghrelin has been shown to inhibit insulin release in mice, rats, and humans. Ghrelin antagonists or genetic blockades of islet-derived ghrelin markedly augment glucose-induced insulin release [
63]. Inhibition of glucose-induced insulin secretion by ghrelin involves direct interaction of ghrelin with ghrelin receptor coupled to novel cAMP/TRPM2 (cyclic adenosine monophosphate/transient receptor potential melastatin 2) signaling in β-cells [
64]. This β-cell unique ghrelin receptor with insulinostatic signaling largely accounts for the systemic effects of ghrelin on circulating glucose and insulin levels. Activation of ghrelin receptor in β-cells inhibits the glucose-induced cAMP and TRPM2 production, and suppresses the glucose-induced [Ca(2+)](i) increase in the β-cell, leading to inhibition of insulin release by β-cells in pancreatic islets [
63,
64].
There are other functions of ghrelin that are worth mentioning. Vestergaard et al. demonstrated that acyl-ghrelin infusion increases thirst sensation in humans, without affecting diuresis and renal sodium excretion [
65]. Ghrelin has been reported to exhibit antidepressant effects [
66]. Moreover, Liu et al. showed that ghrelin promotes neural differentiation of adipose tissue-derived mesenchymal stem cells, through the activation of β-catenin and AKT/mTOR signaling pathways [
66,
67].